Society pays a high price for Alzheimer’s disease. Among medical maladies, Alzheimer’s and other dementias rank second only to cancer in costs of treatment, care and indirect expenses. In fact, the societal expense of Alzheimer’s is no doubt underestimated: The numbers usually don’t include the burden to family members who provide care during the disease’s early stages, marked by modest memory problems. Eventually severe memory loss, other mental impairments and even personality change afflict its victims. And there is very little that modern medicine can do about it.
“Despite decades of study of the basic biology of Alzheimer’s disease and significant pharmaceutical industry efforts to develop therapies, there is no effective therapy available,” write W. Vallen Graham and his coauthors in the Annual Review of Medicine.
Drugs and prevention both wanting
So far, only four drugs have been approved for Alzheimer’s treatment, none remarkably effective. They relieve some of the memory problems to some extent. But they don’t attack the disease at its roots. “There is no clear evidence that any of the currently available drugs modifies primary pathological processes that underlie disease,” Graham and his collaborators write.
As effective drugs for curing or ameliorating Alzheimer’s symptoms have proved elusive, researchers have lately focused more on finding ways to prevent the disease in the first place. But all such efforts, whether in search of prevention or cure, face formidable obstacles.
One major barrier is the lack of clear criteria for diagnosing Alzheimer’s and distinguishing it from other forms of dementia. No easily measured biological substance or indicator can determine who is suitable for clinical trials or gauge a treatment’s success. Substantial research is under way on genetic analyses and brain imaging techniques to identify people for trials while their disease is in its earliest stages, when preventive therapies might be most effective.
In the meantime, dozens of potential drugs are under scrutiny in numerous clinical trials, testing a handful of strategies suggested by current neurobiochemical knowledge of the disease. But applying that knowledge to the hunt for treatments isn’t easy, primarily because Alzheimer’s biology is so complicated. For decades scientists have fingered amyloid protein plaques as well as tangles of a protein known as tau as suspects in Alzheimer’s. Both seem to damage and kill nerve cells. More recently, the inflammatory response from the body’s disease-fighting immune system has been implicated in Alzheimer’s. But it’s unclear which are causes rather than effects of the disease.
The amyloid hypothesis
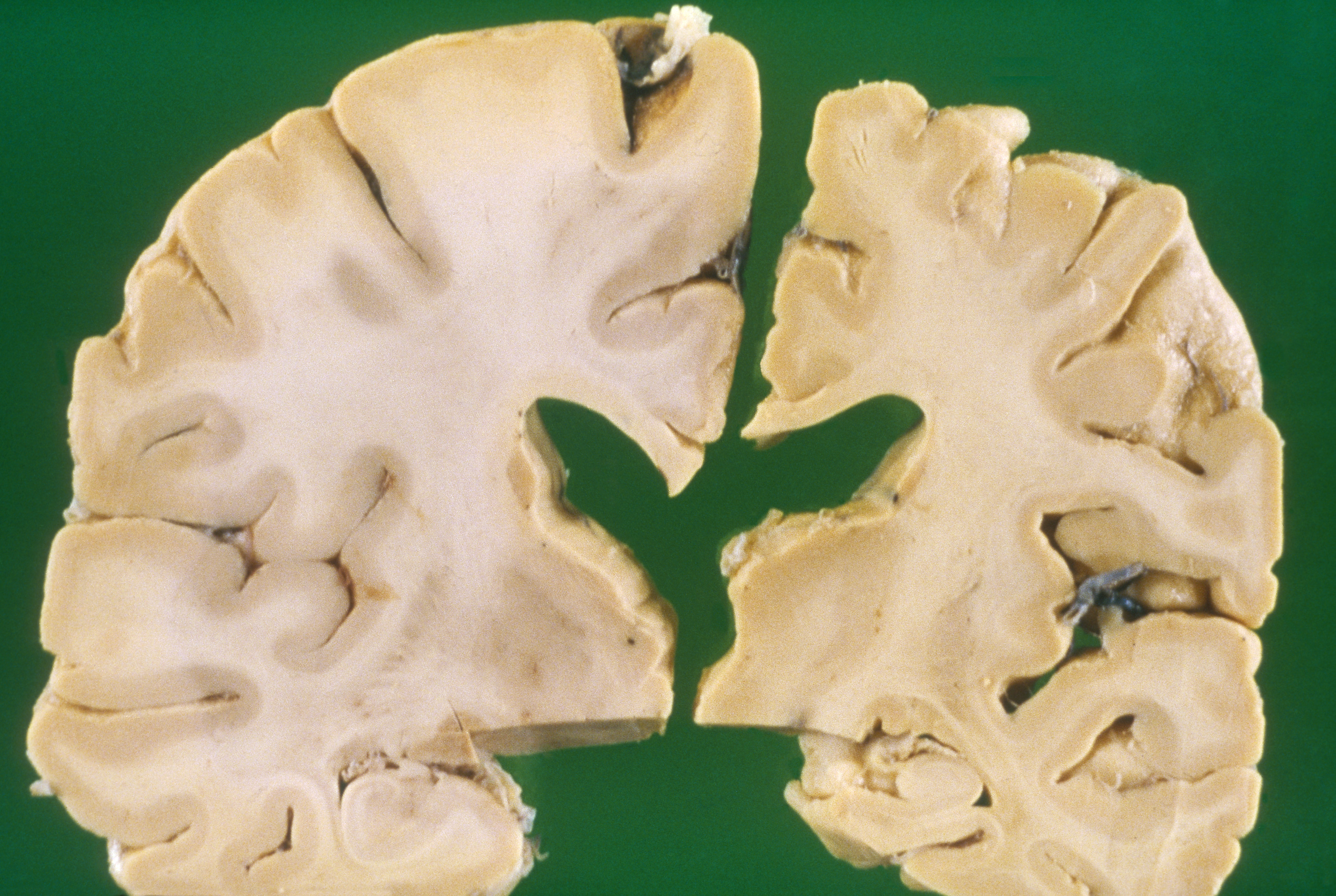
Relative to the brain of a healthy 70-year-old adult (left), the brain of an Alzheimer’s patient of the same age is shrunken (right).
CREDIT: BIOPHOTO ASSOCIATES / SCIENCE SOURCE
Many experts have long suspected that amyloid-beta plaques are the primary culprit. Those plaques are aggregates of amyloid-beta peptides, short chains of amino acids cleaved from the amyloid precursor protein, known as APP. Enzymes called secretases slice amyloid-beta peptides from APP; those peptides then aggregate into fibrils that intertwine themselves into the deadly plaques.
One anti-Alzheimer’s strategy has been to develop drugs that disable the secretases, cutting off the production of the amyloid-beta peptides. So far, this approach has not been especially successful. For one thing, the secretases also serve other important biological purposes, so blocking their action has led to toxic effects such as impairing the immune system, producing gastrointestinal bleeding or damaging the liver.
More recent drug candidates use a different approach in an attempt to modify secretase activity to reduce amyloid-beta production without the damaging side effects. One promising tactic is the use of antibodies against one of the secretases, beta-site APP cleaving enzyme 1, or BACE-1. A clever version of that strategy uses antibodies to block the site on APP where BACE-1 slices; BACE-1 therefore is not be able to cut up APP but remains able to perform its other jobs elsewhere.
Another strategy targets amyloid cleanup rather than its production. Amyloid-beta levels in the brain depend not only on how much is produced from APP, but also on how rapidly the brain’s metabolic vacuum cleaners remove it. Excess amyloid-beta might therefore be caused not by too much production, but by faulty cleanup mechanisms.
Among other anti-amyloid strategies are drugs that boost the production of enzymes that chop up amyloid-beta peptides. Other drugs are being tested for their ability to interfere with various steps in the process of plaque formation.
Especially interesting is the role of chaperone molecules. Such molecules help proteins fold into the proper shapes necessary to do their biological jobs. Animal studies show that several chaperone proteins can deter the aggregation of amyloid-beta peptides. Different chaperones impede different steps in the process leading to plaques, offering multiple opportunities to intervene by enhancing the chaperone’s effectiveness.
Tackling tau
While the most widely watched research has focused on countering amyloid-beta, another protein has also long been implicated as a possible Alzheimer’s culprit. That protein, known as tau, ordinarily stabilizes microtubules, structures that help transport molecules within nerve cells.
In Alzheimer’s brains, tau goes awry. Numerous phosphate groups attach to normal tau which then aggregates into “neurofibrillary tangles” that damage microtubules and kill brain cells. Various research efforts are in progress to find ways to reduce tau production, prevent it from tangling or clear out the tangles.
The small RNA molecule miR-219, for instance, limits tau production; miR-219 is known to be less abundant in the brains of Alzheimer’s patients, and so boosting levels of it offers a possible therapy. Another approach involves activating the body’s immune system to attack phosphorylated tau; two such vaccines using that approach are now in clinical trials.
Brain on inflammation
Besides amyloid-beta plaques and tau tangles, a third feature common to Alzheimer’s is inflammation. Immune cells called microglia typically scavenge the brain to clean up debris resulting from cellular damage. But in an aging brain, microglia can go rogue. Damage to their DNA can alter microglia behavior so that they inflict inflammation rather than respond to it. Excessive microglia activity has been linked both to amyloid plaque formation and the worsening of Alzheimer’s dementia. Finding the genetic markers signaling microglia gone bad offers another avenue to possible intervention strategies.
An immune system–Alzheimer’s connection is especially intriguing, as some research indicates that amyloid-beta itself may play a role in fighting infections. Plaques may ordinarily form as a way to “cage” invading microorganisms, some studies suggest. Perhaps the body’s normal disease-fighting system runs amok in Alzheimer’s and in the process produces excessive amounts of amyloid plaque.
Along these lines, Graham and his coauthors suggest that successful Alzheimer’s therapy may involve treating the entire body rather than just the brain. The ApoE4 molecule, known to raise the risk of Alzheimer’s, is also linked to diabetes, and there is some evidence of an Alzheimer’s link to obesity and insulin resistance as well.
In fact, an insulin-degrading enzyme, which plays a role in clearing out amyloid-beta, may be less available for that job in people with insulin resistance. Some mouse and human studies hint that drugs for fighting diabetes may improve mental function. “Therefore, insulin signaling seems to be a novel therapeutic target for Alzheimer’s disease,” Graham and collaborators write.
The road ahead
While targeting insulin signaling and some of the many other approaches now being tested still seem promising, it is clear that defeating Alzheimer’s will require a much better grasp of the basic biology underlying its devastation. Molecular and cellular details of its pathology are just not fully understood.
Deeper knowledge is needed to develop better systems for classifying people with Alzheimer’s so drug candidates can be better targeted to individuals most likely to benefit. Such focused trials might help researchers better distinguish causes from effects, and perhaps ultimately lead to precise therapies geared to individual patients.
“If there is one overriding lesson to be learned from Alzheimer’s disease drug-development efforts to date,” Graham and his coauthors write, “it is that more basic knowledge about Alzheimer’s disease and other neurodegenerative diseases is still needed.”




