In a small windowless room on a sweltering summer’s day, I find myself face-to-face with an entomological rock star. I’m at the University of Lincoln in eastern England, inside an insectary, a room lined with tanks and jars containing plastic plants and dozing insects. Before I know it, I’m being introduced to a vibrant-green katydid from Colombia.
“Meet Copiphora gorgonensis,” says Fernando Montealegre-Z, discoverer of this six-legged celebrity. The name’s familiar: It’s been splashed across the world alongside photos of the insect’s golden face and miniature unicorn’s horn. The renown of this katydid rests not on its looks, though, but on its hearing. Montealegre-Z’s meticulous studies of the magnificent insect revealed that it has ears uncannily like ours, with entomological versions of eardrums, ossicles and cochleas to help it pick up and analyze sounds.
Katydids — there are thousands of species — have the smallest ears of any animal, one on each front leg just below the “knee.” But their small size and seemingly strange location belie the sophisticated structure and impressive capabilities of these organs: to detect the ultrasonic clicks of hunting bats, pick out the signature songs of prospective mates, and home in on dinner. One Australian katydid has capitalized on its auditory prowess to capture prey in a very devious way: It lures male cicadas within striking distance by mimicking the female part of the cicada mating duet — a trick requiring it to recognize complex patterns of sound and precisely when to chip in.
Awesome? Absolutely. Unexpected? That, too. I’d never given much thought to insect ears until now. Insect eyes and antennae stand out, but ears? Even the eagle-eyed could be forgiven for wondering if insects have them. Yet obviously, some must hear: The summer air is filled with the trills, chirps and clicks of lovelorn crickets and grasshoppers, cicadas and katydids, all trying to attract a mate.

A greater horseshoe bat hunts a moth. The appearance of bats that hunt with the aid of ultrasonic sonar drove the evolution of hearing in many moths and other night-flying insects. Most moths have ears tuned to the frequencies used by bats.
CREDIT: AVALON / PHOTOSHOT LICENSE / ALAMY STOCK PHOTO
Curiosity piqued, I call neurobiologist Martin Göpfert at the University of Göttingen in Germany, who studies hearing in the fruit fly Drosophila melanogaster. Amazing though katydid ears are, he tells me, they’re just one of many with astonishing capabilities: Evolution has made so many attempts at shaping ears, the result is a huge diversity of structures and mechanisms. Most are hard to spot, if not invisible, and in many cases insects produce and sense sounds so far beyond our own range that we overlooked their abilities entirely. But with the advent of new tools and technologies, ever more examples are coming to light.
Sensory biologists, acoustics experts and geneticists are working together to pin down how they all work, how and when they evolved, and why. And thanks to some of this newfound knowledge, and an assortment of fossil insects, there’s even the tantalizing prospect of being able to eavesdrop on the ancient past, adding a new dimension to our understanding of the life and times of some long-vanished animals.
When insects first appeared some 400 million years ago, they were deaf, Göpfert tells me. These ancestral insects went on to diversify into more than 900,000 species, and while most remain as deaf as their ancestors, some gained the means to hear. Of the 30 major insect orders, nine (at last count) include some that hear, and hearing has evolved more than once in some orders — at least six times among butterflies and moths. The 350,000 species of that most dazzlingly diverse group, the beetles, are almost all deaf, yet the few that have ears acquired them through two separate lines of evolution. All told, insect ears arose more than 20 separate times, a sure-fire recipe for variety.
Ear, there and everywhere
Location is the most obvious difference between one insect’s ears and another’s: There are ears on antennae (mosquitoes and fruit flies), forelegs (crickets and katydids), wings (lacewings), abdomen (cicadas, grasshoppers and locusts) and on what passes for a “neck” (parasitic flies). Among moths and butterflies, ears crop up practically anywhere, even on mouthparts. The bladder grasshopper has an abundance of ears with six pairs along the sides of its abdomen. Praying mantises have a single, “cyclopean” ear in the middle of their chest.
This anywhere-goes approach might seem a little weird but there’s a simple explanation: In every case where an insect ear evolved, the starting point was an existing sensory organ: a stretch detector that monitors tiny vibrations when neighboring body segments move. Those detectors occur throughout the insect body but evolution typically only modified a single pair — apparently, almost any pair — to perceive the airborne vibrations generated by sound.
From there on, each new attempt to forge ears went even further in its own direction as other structures were co-opted and reconfigured to capture, amplify and filter sound, extract the relevant information and convey it to the nervous system. In mosquitoes and fruit flies, sound causes fine antennal hairs to quiver. Most other hearing insects have “eardrums”: thin, membranous patches of exoskeleton that vibrate when sound waves hit. Some eardrums are backed by air-filled acoustic chambers, others by fluid-filled ones. The number and arrangement of sensory cells that detect and decode those vibrations — and the neurons that send the signals to the brain — also vary from ear to ear. So while some moth ears function with just one or two neurons (making moths the most rapid responders), a male mosquito’s ear has around 15,000 (making it exquisitely sensitive).
Some ears are relatively simple; others have extra bells and whistles linked to their lifestyle. Take the parasitic fly Ormia ochracea, which deposits its larvae on a particular species of cricket after identifying and locating it from its characteristic call. The fly’s ears sit side by side on its “neck” and are theoretically too close together to pinpoint its target. Yet they take the prize for accurate location, thanks to an elastic band connecting the eardrums so they rock up and down like a seesaw, ensuring sound hits one ear fractionally later than the other.
Katydid ears, as so neatly demonstrated by Montealegre-Z and his colleagues, are unique both in their complexity and their similarity to a mammal’s. Using a micro-CT scanner, the scientists reconstructed the insect’s entire hearing system, discovering two previously unknown organs in the process. The first is a small, hard plate behind the eardrums; the second, a fluid-filled tube containing a line of sensory cells. Through painstaking investigation that included shining lasers at the eardrum and recording the light bouncing back, the team showed that the small plate transmits vibrations in the insect’s eardrum to the fluid in the tube — the same role played by the bones in our middle ear. The signal then travels in a wave along the tube and over sensory cells tuned to different frequencies — making this organ a miniature, uncoiled version of our own, snail-shaped cochlea.
The team has now gone on to show why female katydids are so good at finding a mate in the dark, even though their ears are close together (not so close as those of the parasitic Ormia, but near enough to make pinpointing sound a sizeable challenge). Our own ears lie on either side of our (large) heads and are far enough apart for a sound to reach them at different-enough times and loudness for the brain to compute and locate the source.
Katydids solved the problem (again, in a unique way) by enlarging a breathing tube that runs from a pore in the side of the chest to the knee; sound reaches the eardrums both from outside the body and from the inside via the tube. Montealegre-Z and his colleagues showed that sound travels this inner, back route more slowly — so each sound hits the eardrum twice, but at slightly different times, dramatically improving the insect’s ability to locate the source.
$[$PB_DROPZONE,id:donate-promo$]$The katydid’s remarkable ears haven’t yet given up all their secrets, and Montealegre-Z’s team is now trying to pin down how the receptors in the insect version of the cochlea pick out different frequencies. The star of this study is Phlugis poecila, a “crystal” katydid named for its transparent outer cuticle, a feature that allows the team to record and measure processes as they happen. “We’ll be able to watch hearing at work and see processes never seen before,” Montealegre-Z says.
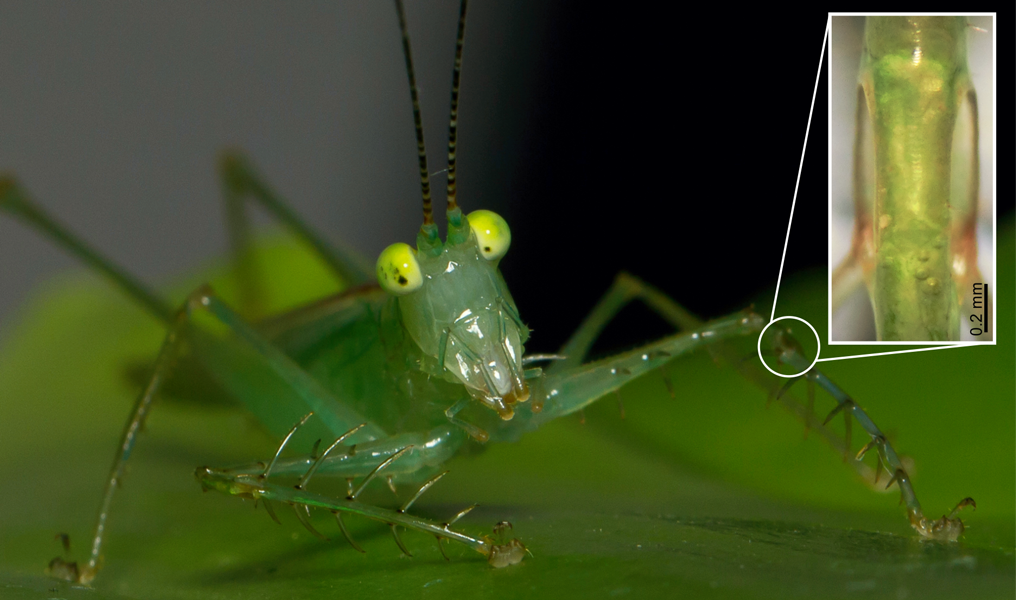
Crystal ear-gazing: Phlugis poecila, a crystal katydid from the rainforests of Colombia, has such a transparent outer cuticle that scientists can see right through its eardrums (inset). By shining lasers into its ears they can record activity of the inner ear as it analyses the frequency of incoming sound.
CREDIT: FABIO SARRIA-S
If how insects hear varies enormously, so does what they hear. Mosquito ears are good for maybe a meter; the many-eared bladder grasshopper can hear from a kilometer or more away. Cricket ears detect low frequencies; mantis and moth ears are tuned to ultrasound, way beyond anything humans (or their dogs) can hear. Still others, such as a katydid’s, have broadband hearing. “Insects only hear what they need to hear,” says Göpfert. “And evolution provided what was necessary.”
But what drove evolution to turn stretch receptors into ears in the first place, and so bring sound to the insect world? That’s a question still on many entomologists’ minds. A reasonable guide is how insects use their ears today, but it’s only a guide, since an ear originally acquired for one purpose might easily have been co-opted over the eons to serve another. One thing’s certain: As biologists investigate more insect groups in greater detail, some long-held notions may bite the dust.
An ear for danger
In modern insects, one of the primary functions of ears is to hear the approach of a predator in time to take action and avoid it. For night-flying insects, the greatest threat comes from insectivorous bats that detect and track prey with ultrasonic sonar, and so their hearing is tuned to the frequencies of the bats’ echolocating clicks. The insects then respond with characteristic moves to escape the sonar beam: sharp turns, loop-the loops, air-to-ground power dives. Certain tiger moths even jam the bat sonar with clicks of their own. Experiments have shown that bat-detecting ears dramatically improve an insect’s prospects of surviving attack: In one study, mantises escaped 76 percent of bat attacks, but that number fell to 34 percent when they were deafened.
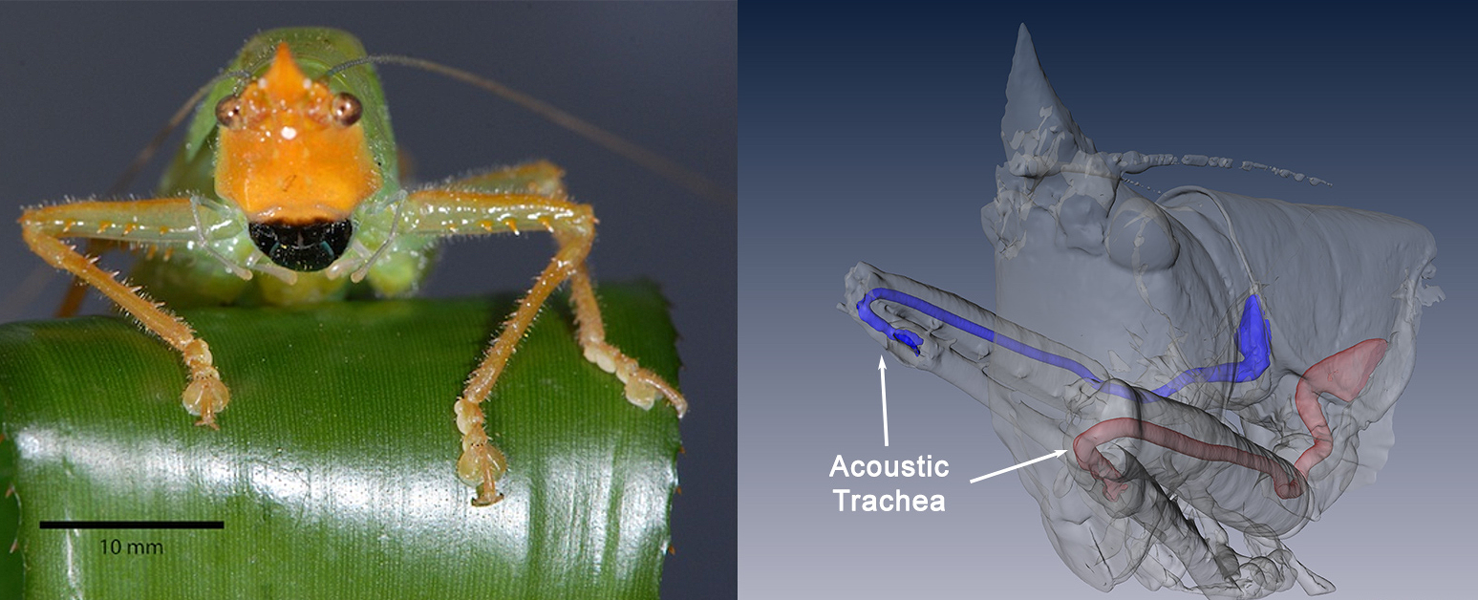
Katydids can pinpoint the source of a sound because every sound hits the eardrums twice, once from outside the body and once from inside. This micro-CT reconstruction (right) of Copiphora gorgonensis (photo, left) shows the inside route. Breathing tubes have been modified to form a sound channel that runs from a pore in the side of the chest, along the leg to the rear of the eardrums, which lie just below the “knees.” Sound travels the inside route more slowly, so it reaches the eardrum slightly later.
Editor's note: This caption was updated November 28, 2018 to clarify details of how the katydid hears.
CREDIT: LEFT, DANIEL ROBERT & FERNANDO MONTEALEGRE-Z. RIGHT, THORIN JONSSON
If predation is a powerful driver of evolution, so, too, is sex. And sound is an efficient way for an insect to identify itself to prospective mates: Sound travels well, works in the dark and provides the means to develop signature songs and private communications that no one else can hear.
So, successful sex or survival? Which lies behind whose ears?
In some cases, researchers are reasonably sure. Cicadas seem to have evolved hearing for mating purposes: Only singing species have ears and they are sensitive only to their own low-pitched songs. For moths, bats were the trigger. Lepidoptera have been around some 150 million years, yet no moths had ears before echolocating bats arrived on the scene about 60 million years ago. And many of the eared moths are sensitive only to the frequencies employed by their local bats — strong evidence that the ears evolved as bat detectors.
What, though, to make of the mantis, owner of the cyclopean ear? Today, mantises seem to use their ears exclusively as bat detectors. But entomologists now have vast amounts of data on the varied anatomy of mantis ears and an accurate DNA-based mantis family tree, from which they traced the original mantis ear. It belonged to a species that lived 120 million years ago, rather earlier than those sonar-guided bats. There’s growing evidence that predators other than bats might have spurred the evolution of their ears and those of some other insects — perhaps reptiles, or birds, or early mammals. Animals moving through the undergrowth, pattering over rocks or landing on a leafy branch are rarely silent. The noises they make include audible and ultrasonic elements.

The European praying mantis (Mantis religiosa) has a single ear located in a deep groove that runs down the middle of its chest. At the sound of a hunting bat, mantises make dramatic moves to evade capture. Yet these ears originated many millions of years before bats existed.
CREDIT: WILDLIFE GMBH / ALAMY STOCK PHOTO
Flying birds, which have existed for 150 million years, are increasingly seen as contenders. In groundbreaking research, Canadian biologists recorded sounds generated by the beating wings of chickadees and eastern phoebes as they moved in on insect prey, and found that the wing beats included a wide range of frequencies that insects can detect, from low-pitched sounds audible to cicadas, butterflies and grasshoppers, to ultrasonic sounds picked out by moths and mantises.
And what of the katydids, possessors of the most ancient ears of all? Modern katydids use their ears both in communication and as bat detectors. But the katydid sound-producing apparatus can be traced back through the fossil record to an early type of ancestor that lived 250 million years ago, well before bats did. So the prevailing theory up till now has been that the evolution of katydid ears took some turns. The ears’ initial function was to enable katydids to hear one another, and later on, the thinking goes, those ears were co-opted to serve as bat detectors. This led to the extension of their hearing from the audible range (below 20 kHz) to the ultrasonic (beyond the reach of human ears) — and that, in turn, allowed evolution of the more complex, higher-pitched songs that katydids exhibit today. Today, only a minority of katydids sing in the audible range, while about 70 percent have ultrasonic songs and a few have extraordinarily high-pitched songs. The record holder, so far, is the recently discovered Supersonus aequoreus, which calls at an astonishing 150 kHz.
But is that story right? To get at the answer, scientists needed to know what katydids were hearing in the distant past, and that meant taking a close look at katydid fossils. The fossilized ears are not themselves very informative: They are rare and their structure hard to make out. But there’s another way of getting at hearing: from the detailed anatomy of the sound-producing file-and-scraper apparatus on fossilized katydid wings. “Those structures are much larger and clearer, and we can use them to recreate the sound they made very accurately,” says Montealegre-Z — and from that, infer what katydids must have heard.
Blast from the past
In 2012, Montealegre-Z and fellow bioacoustics expert Daniel Robert at the University of Bristol made headlines when they used this approach to reconstruct the song of a katydid from Jurassic times, a sound unheard for 165 million years. What made that possible was the discovery of a Chinese fossil katydid with almost perfectly preserved wings. Archaboilus musicus, as the extinct insect has been named, would have “sung” musical songs at frequencies around 6.4 kHz, sounding more like a cricket than a modern katydid. That fits nicely with the story that katydids first evolved hearing to communicate.
Song from the distant past: By analyzing the file-and-scraper apparatus on a fossilized katydid’s wings, scientists reconstructed the call of a katydid from Jurassic times — 165 million years ago.
CREDIT: PNAS / GU ET AL. VIA YOUTUBE
Since then, though, the team has been studying more fossil katydids, and what they are finding suggests that the theory might need an overhaul. It seems that some ancient katydids were using ultrasound long before bats existed, says Montealegre-Z. Katydids also hear a much wider range of frequencies than they’d need just to hear themselves. To his mind, this suggests that their ears first evolved not for singing but, much like mantises, for self-preservation. “I think their ears evolved to hear predators,” he tells me. “Predators make a diversity of sounds and so ears must be able to pick them out.”
If studies like these are helping to unravel the evolutionary history of insect hearing, they also promise something more: the opportunity to eavesdrop on the ancient past and gain new insights into insect behavior. They’ve also made me impatient for next summer and the chance to explore the rich insect life of the gently rolling chalk hills hereabouts with new eyes — and ears, especially ears.
In summer, the air over the Sussex Downs is alive with a symphony of insect sound as grasshoppers and katydids chirp, buzz and click in their quest for love. If I strain my ears to the limit, I might be able to pick out the sewing-machine rattle of a great green katydid or the soft hissing song of a conehead, and if I’m very lucky, perhaps even the rapid-fire clicks of the wart-biter, the UK’s rarest katydid. But how much more will I be missing? I’d give a lot to have ears that can pick out the songs and sounds scientists are piecing together, but that insects alone can hear.




