Zooming in on the brains of babies
New tools are helping neuroscientists investigate why early life is such a crucial time for neural development
Support sound science and smart stories
Help us make scientific knowledge accessible to all
Donate today
Many of our defining traits — including the languages we speak and how we connect with others — can be traced back at least in part to our earliest experiences. Although our brains remain malleable throughout our lives, most neuroscientists agree that the changes that occur in the womb and in the first few years of life are among the most consequential, with an outsize effect on our risk of developmental and psychiatric conditions.
“Early on in life, the brain is still forming itself,” says Claudia Lugo-Candelas, a clinical psychologist at Columbia University and coauthor of an overview of the prenatal origins of psychiatric illness in the Annual Review of Clinical Psychology. Starting from a tiny cluster of stem cells, the brain develops into a complex organ of roughly 100 billion neurons and trillions of connections in just nine months. Compared to the more subtle brain changes that occur later in life, Lugo-Candelas says, what happens in utero and shortly after birth “is like building the house, versus finishing the deck.”
But just how this process unfolds, and why it sometimes goes awry, has been a hard mystery to crack, largely because so many of the key events are difficult to observe. The first magnetic resonance imaging (MRI) scans of baby and fetal brains were taken back in the early 1980s, and doctors seized on the tool to diagnose major malformations in brain structure. But neuroimaging tools that can capture the baby brain’s inner workings in detail and spy on fetal brain activity in pregnant moms are much newer developments. Today, this research, coupled with long-term studies that follow thousands of individual children for years, is giving scientists new insights into how the brain develops.
These advances have propelled researchers to a different stage than they were in even five years ago, says Damien Fair, a neuroscientist at the University of Minnesota who studies developmental conditions like autism and attention deficit hyperactivity disorder (ADHD).
Until recently, a major challenge has been that, unlike an adult, a fetus or newborn baby won’t lie still inside a brain scanner. Buoyed by amniotic fluid, a fetus constantly shifts position, and newborn babies love to wriggle around, checking out their environment. In the past, researchers and clinicians often had to do multiple time-consuming, expensive scans to get a good image. They sometimes sedated children and pregnant moms to reduce movement, an approach that alters brain function and may have health risks.
But new imaging and computational techniques that reduce distortions caused by fidgeting — including software developed by a company cofounded by Fair — have made it easier to collect data from babies and fetuses. And that has invigorated the field.
Watch the replay of this event held on March 23, 2023.
Peering into prenatal brain development
The new work is starting to reveal what typical brain development looks like and hint at how atypical conditions like autism and ADHD may arise. In a first-of-its kind study in 2017, for example, a team of researchers led by pediatric neuroscientist Moriah Thomason, now at New York University, used functional magnetic resonance imaging (fMRI) to investigate patterns of neural communication among brain regions in 32 fetuses. Half of the pregnant women were at high risk of early delivery and 14 of the babies ultimately were born prematurely.
Premature birth is a known risk factor for cognitive and emotional issues later on. But it has been difficult for scientists to determine whether this is due to the trauma of premature delivery, which often involves brain injury and oxygen deprivation, or to preexisting brain differences that start in the womb.
Thomason’s study provided the first evidence that the problems start in utero.
As fetuses, the preemies-to-be that were scanned by her team had brain activity that suggested weaker communication between various brain regions compared with fetuses that ended up being carried to term. Most strikingly, the scientists found altered neural communication in networks that eventually support language, including a language center on the left side of the brain.
Researchers have since found more evidence for prenatal brain disruption in preemies. In 2021, for example, another group found that 24 prematurely delivered infants had lower brain volumes and less cerebrospinal fluid while still in utero, compared with a group of infants carried to term. And a variety of studies have found that women who delivered prematurely had high levels of inflammation caused by bacterial or viral infections in the amniotic fluid and placental tissues.
The findings add to growing evidence that inflammatory events during pregnancy can alter fetal brain development. Large-population studies, for example, have shown that mothers who have had a severe infection during pregnancy are at a slightly elevated risk of having an autistic child, although it’s not yet clear that prenatal infection alone can actually cause autism.
Lugo-Candelas’s research focuses on how a pregnant woman’s perceived stress, life events, depression and anxiety may affect early brain development. A number of studies have found that high maternal anxiety and depression during pregnancy are associated with a twofold increase in the risk of the child developing a mental disorder later in life. If the risks start earlier in development, “that also means there’s a chance to intervene earlier than we thought,” she says. But, Lugo-Candelas adds, scientists are still working to untangle the mechanisms behind that increased risk, what stressors might have the most impact, and when and how to intervene.
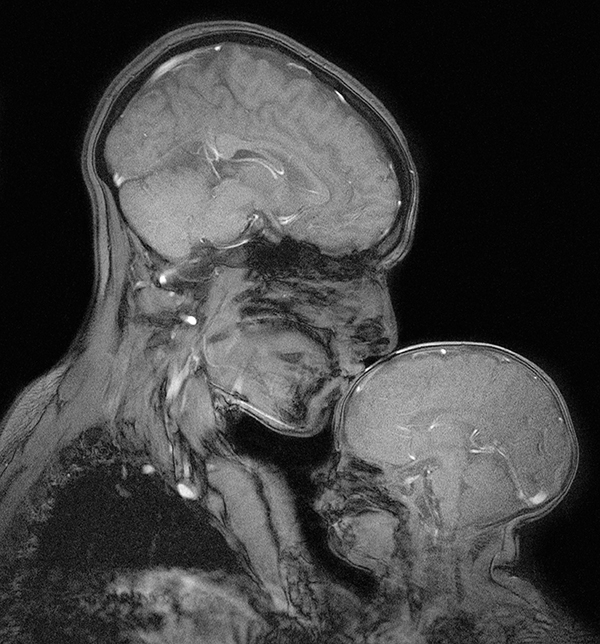
An MRI scan shows MIT neuroscientist Rebecca Saxe kissing her 2-month-old son. Advances in imaging software have allowed researchers to better study the changing brains of babies.
CREDIT: REBECCA SAXE, BEN DEEN & ATSUSHI TAKAHASHI / MIT, ATHINOULA A. MARTINOS IMAGING CENTER AT THE MCGOVERN INSTITUTE FOR BRAIN RESEARCH / MIT
Moreover, like many other risk factors in pregnancy, there’s no one thing that leads to psychiatric illness or developmental problems, says Lugo-Candelas. “It’s a collection of tiny risks.” She emphasizes that there’s nothing rigidly deterministic about any of these early exposures or experiences. “You can have children that are exposed prenatally to a bunch of the things that we think could increase risk for a psychiatric disease, and then have a child that doesn’t have a disorder at all and will never have it.”
That complexity speaks to one of the greatest challenges of studying the developing brain: the fact that similar outcomes, such as autism or schizophrenia, can have many underlying neurological causes. Some people with autism have increased connectivity between certain brain regions compared with the neurotypical population, for example — but others have less. There’s no single neural signature for the condition.
Brain connections as ‘neural fingerprints’
Fair’s approach to this problem has been to identify what he calls “functional fingerprints,” patterns — unique to each individual — in how different brain regions communicate with each other when a person is at rest inside an fMRI scanner.
He first observed these neural fingerprints in adults in 2014, and went on to show that children have them too. The patterns are surprisingly consistent within families, even across generations, he and his colleagues have found, suggesting that certain types of brain connectivity are at least partially inherited.
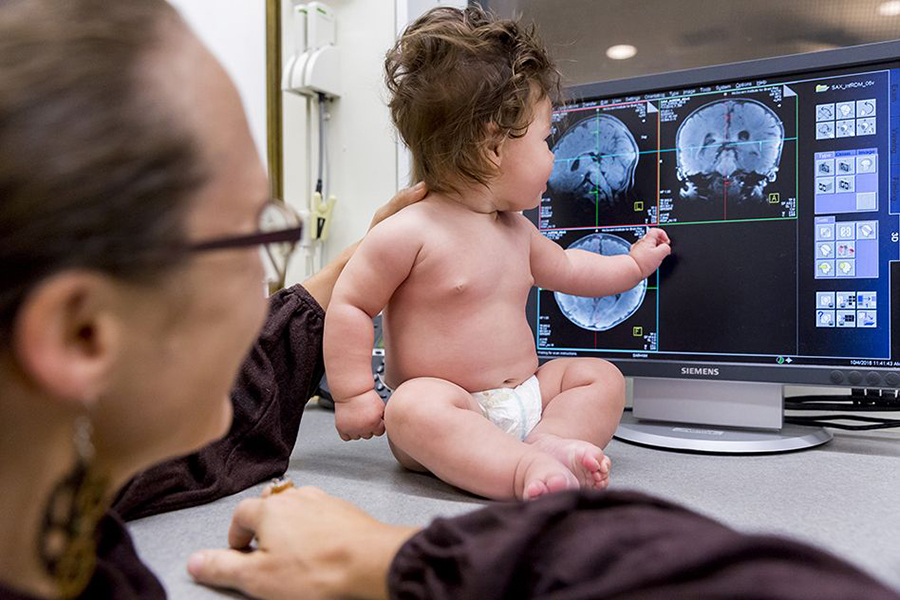
Neuroscientists at MIT have made their brain imaging set-up more baby-friendly to learn more about early development. Using an adapted MRI scanner, researchers can image infants’ brains as the babies watch movies with different types of visual stimuli.
CREDIT: PHOTO BY CAITLIN CUNNINGHAM
Last year, he published evidence that even eight-month-old babies have these neural fingerprints — and that certain elements of the fingerprint, such as the amount of crosstalk between regions involved in functions like attention and movement, can predict an infant’s precise age, down to a few months.
Meanwhile, Thomason’s fMRI studies of the fetal brain suggest that these distinct connectivity patterns emerge in the second and third trimester, including in neural circuits that eventually govern learning, memory and emotion. Thomason and others are now using neuroimaging to investigate how a variety of prenatal experiences — ranging from maternal Covid-19 infection to cannabis use — affect how these circuits develop.
The fact that scientists can detect these distinct brain activity patterns so early suggests to Fair and others that much of what makes us who we are is already in place by the time we’re born, even though we’ll continue to be shaped by our experiences and exposures throughout life. Because every baby’s brain is shaped by so many different factors, however, researchers are going to need long-term imaging data from thousands of children to get a robust understanding of what “typical” development looks like, Fair and colleagues argue in the 2021 Annual Review of Developmental Psychology.
Eventually, imaging tools could help clinicians and researchers monitor how a baby’s brain is developing, spot signs of future trouble and develop earlier personalized interventions and treatments for conditions like autism, Fair adds.
In the meantime, Lugo-Candelas thinks that we already know enough to take action. “I feel pretty confident that interventions that effectively minimize distress in pregnancy, like paid maternal leave, are going to be beneficial for the next generation,” she says. She notes that could lead to better outcomes in school and other areas, like mental health, that ripple across the lifespan. “I just don’t think we’ve done a really good job yet at measuring what those outcomes look like, or the mechanisms that lead to them.”
10.1146/knowable-032023-1
TAKE A DEEPER DIVE | Explore Related Scholarly Articles




