Growing up in North Dakota, Ken Libbrecht was no stranger to snow. But it took a move to southern California — to the California Institute of Technology, where he is a physicist — to make him appreciate the science of all those frozen flakes he witnessed as a kid.
While researching crystal growth, Libbrecht became fascinated by the physics of ice and soon turned himself into the world’s foremost expert on snowflakes. On wintertime trips to Michigan, Ontario and other points north, he began catching and photographing ice crystals through a microscope. In a laboratory in sunny Pasadena, he built a cold chamber where he could grow his own flakes.
Over the years Libbrecht has amassed countless examples of classic six-branched snowflakes, as well as plates, columns, prisms and other frosty forms. In the Annual Review of Materials Research, Libbrecht explores the factors that drive the growth of ice crystals — and transform simple water into crystalline beauty.
Surprisingly, there is much physics yet to be learned. “When I started looking at ice I was shocked at how little had been done in the last 50 years,” he says. “Here’s this phenomenon that literally falls out of the sky, and we don’t understand how it works.”
Each snowflake is a crystal of ice that condenses from water vapor in the atmosphere. But depending on the temperature, humidity, wind and other factors where it forms, each flake grows at different rates and thus into a different pattern than those that came before it. Just by watching the types of flakes that fall — whether flakes, plates, columns or some other shape — Libbrecht can pinpoint the temperature and humidity in the clouds above.
In his laboratory, Libbrecht explores scientific questions such as how interactions between air and the growing crystal affect its ultimate shape. But he also dabbles in the artistry of snowflakes — for instance, by growing them much larger than nature can in order to generate new crystal patterns. (An ordinary snowflake might be 3 millimeters across, but Libbrecht is hoping for as much as a centimeter with his planned “uber-snowflakes.”)
It’s this combination of physics and splendor that keeps drawing Libbrecht back to snowflakes. “There’s complexity and symmetry together, which is what makes them really beautiful,” he says. “The scientific side is fascinating, but the artistic side is fun too.” Libbrecht’s snowflake photographs have graced a set of U.S. postage stamps, and he even scored a consulting gig on the Disney movie Frozen. There he helped ensure that every digitally rendered snowflake that floated down on Elsa, Anna and their friends had six sides — not eight, as snowflakes are sometimes wrongly shown.
“In its own small way it made the movie better,” Libbrecht says. “It’s OK to conjure snowflakes out of your fingertips. But they have to look like real snowflakes.”
Ice-crystal basics

CREDIT: K. LIBBRECHT
Every snowflake begins with a tiny heart of ice, created when a droplet of water in a cloud freezes around a microscopic dust particle. This process typically happens between about –6 and –15 degrees Celsius (below the freezing point of water), the range in which dust particles serve as a nucleus for water droplets to freeze. Next the seed crystal starts to capture molecules of water vapor that are floating near it. They freeze onto the crystal and cause it to grow. The crystal continues to get bigger for roughly half an hour, until it becomes so heavy that it drops out of the cloud and no longer has additional water vapor to feed it. A single ice crystal formed this way is known to scientists as a snow crystal. To the rest of us, it’s a snowflake.
Many facets

CREDIT: K. LIBBRECHT
One key process shaping snowflake growth is known as faceting. This hexagonal prism has beautifully sharp facets, viewed here from the side. The structure results from the way water molecules attach to the initial seed crystal. Each water molecule wants to grab on to as many other molecules as possible, and so they naturally self-organize into a pattern that maximizes contact between the molecules and results in six smooth facets. (Start laying pennies on a table, each touching as many other pennies as possible, and you will end up with a similar hexagon-shaped pattern.) Sometimes the snowflake will continue to grow primarily through faceting, producing a large hexagonal crystal like the one shown here.
Hexagonal beauty

CREDIT: K. LIBBRECHT
A second key process that dictates how snowflakes grow is known as branching. Consider an initial seed crystal in the shape of a hexagon. Water molecules prefer to attach to rough surfaces, and the corners of the hexagon are relatively rough. So water vapor often begins accumulating on each of the six corners. What starts out as a small bump on each corner soon becomes a larger bump, and then the beginning of an entire branch of ice. Each branch retains the characteristic 60-degree angle that reflects the molecular lattice of water.
Changing conditions

CREDIT: K. LIBBRECHT
The unlimited variety of snowflakes traces back to the fact that each is born and grows in a unique set of circumstances. The amount of water vapor available in the atmosphere, and the temperature through which the crystal falls as it grows, dictate the one-of-a-kind appearance of the snowflake that forms. Even snowflakes that fall side by side in the same weather system show variation.
Higher humidity leads to faster growth and more elaborate branching; the biggest and most intricate snowflakes, known as dendrites, form when humidity is relatively high and the temperature is around –15 degrees Celsius. Note how the stubby side branches in this fast-growing crystal are not perfectly symmetrical on either side of the six main branches. These differences reflect changing conditions in the time the crystal was forming.
Stellar plates

CREDIT: K. LIBBRECHT
Beyond the six-branched beauties, snowflakes come in a variety of other shapes and sizes. This crystal is an example of a stellar plate. Platelike crystals form at two distinct temperatures: around –2 degrees Celsius and around –15 degrees Celsius. They are extremely sensitive to small changes in environmental conditions — add a little extra humidity and they begin to grow into a more dramatic dendrite.
Changing growth conditions can leave behind ripples, like those seen near the center of this crystal, or ridges like the outlines on the six outer panels. A typical snowflake grows in just 30 minutes, and these complex patterns reflect changes it experienced in that mere half hour.
Columnar crystals

CREDIT: K. LIBBRECHT
Less dramatic than their many-branched cousins, column-shaped ice crystals commonly grow long and slender, like an icy wooden pencil. The ends of the column typically grow faster, resulting in the center of the face being left behind and the column being hollowed out at each end. Eventually the center stops growing altogether. This crystal displays small conical bubbles at each end, probably reflecting a temperature change that happened as the ends of the column hollowed out.
Columns typically form at around –5 degrees Celsius, just below the freezing point of water. When humidity is high they can grow up to 3 millimeters long, in slender shapes known as needle crystals. Clusters of the long skinny crystals are common and can be seen with the naked eye, looking like fragments of white hair falling through the sky.
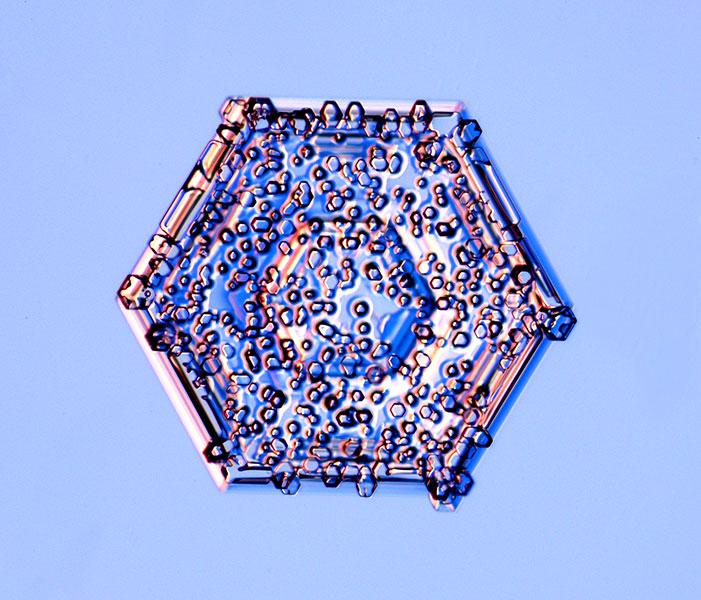
Crystals on crystals
CREDIT: K. LIBBRECHT
After ice crystals form, they often run into other droplets of water vapor that freeze on the crystal structure to form a coating known as rime. Sometimes the rime is so thick that the crystal appears hugely fluffy; other times it forms a ragged rim, as if rolled in a coating of decorative sugar.
Here, the rime particles have taken on an additional aspect. They collided and froze to a large hexagonal crystal, and then began to grow some more. The tiny rime particles are themselves hexagonally faceted, in an orientation that matches the sides of the large crystal. This pattern indicates that the molecular structure of the water in the rime particles has aligned with that of the underlying crystal. They have merged into a single crystalline entity.
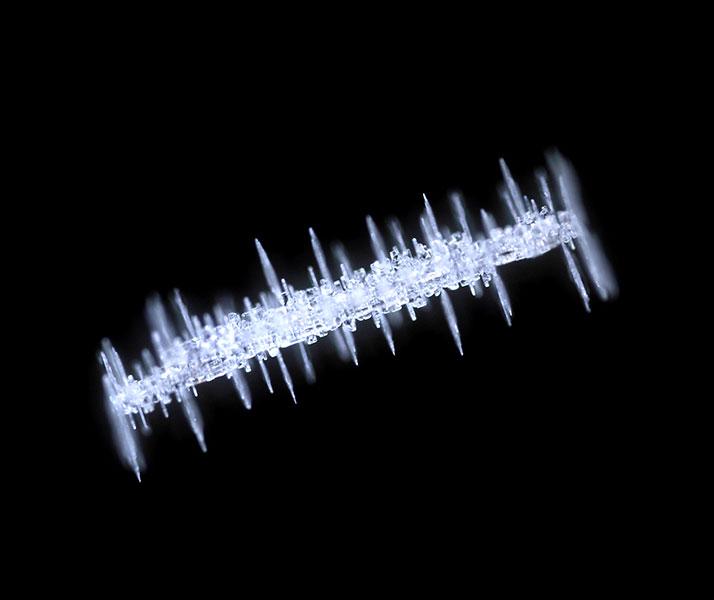
A complex history
CREDIT: K. LIBBRECHT
“I’ve only seen crystals like this once,” says Libbrecht, and it happened outside of Houghton, Michigan. This odd-looking snowflake started life as a very thin columnar crystal, almost like a needle. It then ran into some water droplets that froze and coated it with rime, creating little blobs of ice all along the column.
Next, the crystal drifted to a different part of the cloud where temperatures dropped, perhaps to –10 or –15 degrees Celsius where plates can begin growing. The rime droplets then began growing a bunch of plates outward, which appear as crosswise plates perpendicular to the main crystal. “That’s pretty unusual for a crystal to have that kind of history,” Libbrecht says. “All of a sudden these crazy things appeared for 10 minutes, and that was the end of that.”
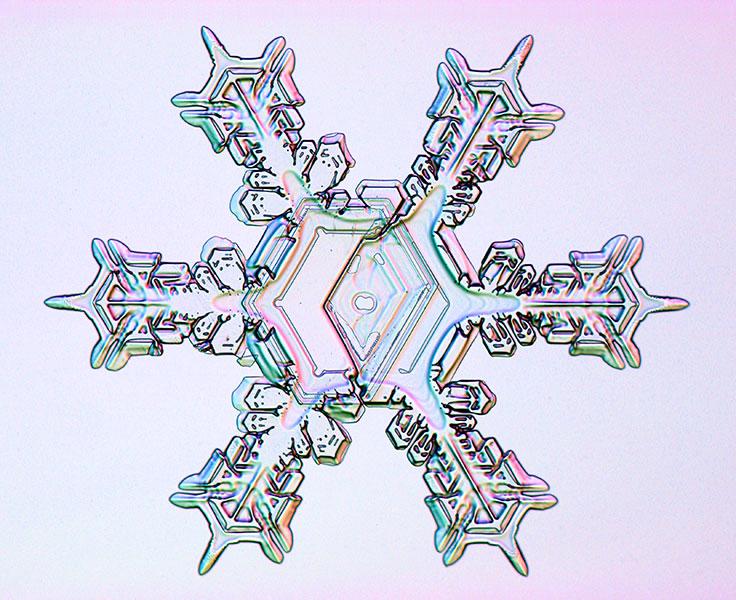
Less than perfect
CREDIT: K. LIBBRECHT
Although this crystal may seem perfect in its outer reaches, the deformation at its heart betrays its complex history. The snowflake began growing as a simple hexagonal prism, with two plates capping each of its ends like a spool for thread. Soon, conditions shifted to favor the growth of one side of the plate on one end, and the other side of the other.
The left-hand side of this crystal snatched water vapor from the air and grew outward, while the right-hand side did so in the opposite direction. The thin column that still connects the two plates can be seen at the very center of the crystal as a tiny oval.
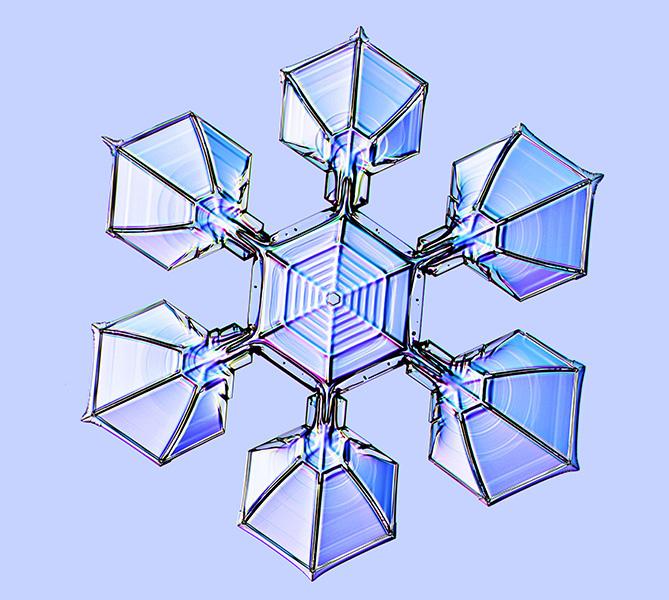
Made by design
CREDIT: K. LIBBRECHT
The heart of this snowflake betrays its artificial origin, with a spiderweb-type pattern that would never be found in nature. Libbrecht created this synthetic snowflake by starting with a tiny seed crystal of ice in a cold chamber. He then fed it water vapor molecules while also tweaking the surrounding temperature. As the ice crystal grew, it retained an imprint of the changing temperature — the spider web — much as tree rings retain a record of changing environmental conditions.
Eventually the corners of the spider web sprouted branches, which in turn generated plates at their ends. The entire process to make this crystal took nearly two hours, much slower than the minutes it usually requires to produce a natural snowflake. Synthetic crystals made side by side can be virtually identical.




