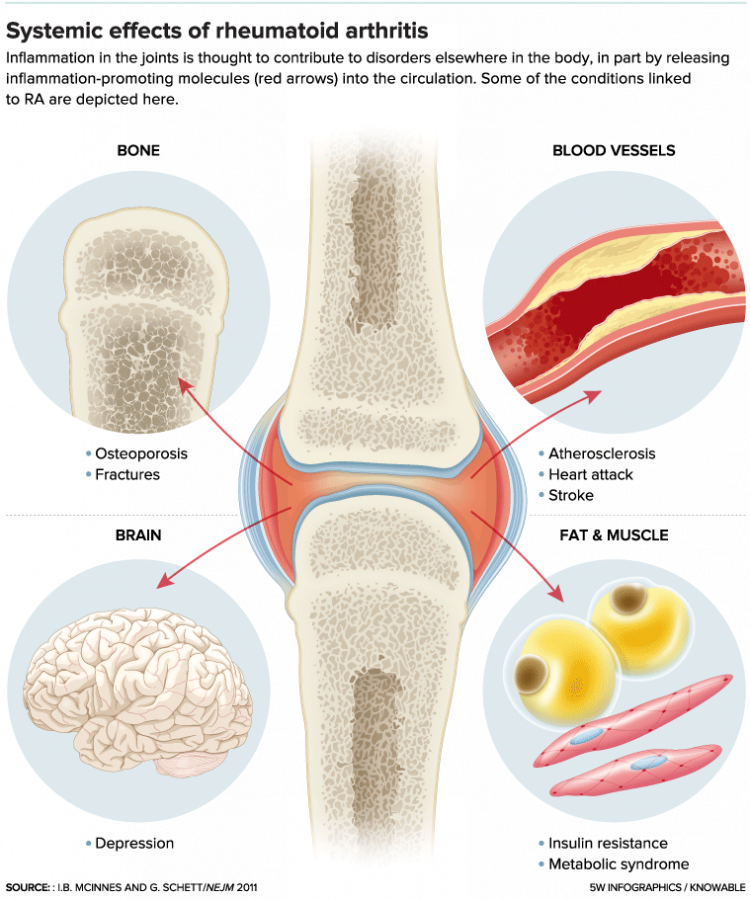
Rheumatoid arthritis leaves a visible mark on the bones of its sufferers as it gnarls and swells knuckles and other joints. It also has invisible systemic effects. Scientists have made enormous advances in understanding its causes and how to treat it, but much about its origins remains murky. It seems to result from a difficult-to-pin-down cascade of genetic and environmental hits that result in autoimmunity and chronic inflammation. And it may not even be a single disease.
Various drugs slow or halt the inflammatory process, which involves excessive activity by immune cells. Scientists now know that limiting inflammation as much and as early as possible can reduce long-term disability and complications. But people with the disease differ in how well they respond to the available medicines. In an online survey, more than half of respondents reported that RA often interfered with sleep, exercise, work, family responsibilities and social life.
Researchers are now trying to understand the variability in disease control. It may be that there are different forms of the disease, involving different combinations of genes and perhaps different triggers. Subsets of patients may need different treatment plans to halt the inflammation and joint damage.
What causes RA?
Rheumatoid arthritis arises from a combination of genetic susceptibility and environmental influences.
Genes: Twin and other studies suggest that genes account for 40 to 60 percent of risk. In addition to certain variants of genes involved in immunity, particularly the HLA-DRB1 gene, scientists have linked more than 100 other common gene variants to RA. Among other effects, HLA-DRB1 variants and their ilk could cause the immune system to overreact to certain of the body’s own proteins, producing antibodies that target the self. Such autoantibodies can fuel inflammation.
For a long time, it was unclear what proteins were targeted in people with RA. It’s now known that the autoantibodies most characteristic of the disease are ACPAs, which home in on proteins that have undergone a chemical change known as citrullination, and RF (rheumatoid factor). Twenty to 30 percent of patients with the symptoms of RA show no evidence of these autoantibodies in their blood, however. These “seronegative” individuals tend to have less bone and joint destruction. Their underlying disease is less studied and may well represent a distinct pathway that results in similar symptoms.
$[$PB_DROPZONE,id:donate-promo$]$Environmental influences: Smoking increases the risk for RA. Obesity and air pollution have also been suggested as possible risk factors. Gum disease and certain bacteria in the gut may trigger joint inflammation. Scientists are still exploring how processes in the lungs, gums and gut could spur disease.
In the healthy joint (left), the capsule surrounding the bones and cartilage is lined by a thin membrane called the synovium. The inner part of this membrane contains cells called synoviocytes that produce a lubricant. In RA (right), the synovium develops an overgrowth of synoviocytes and attracts a rogue’s gallery of immune cells (macrophages, dendritic cells, T cells, B cells, plasma cells and mast cells) that usually fight disease. Together, these cells and their secretions — including signaling proteins known as cytokines — lead to pain and swelling. They can also activate bone-degrading cells called osteoclasts and can progressively damage bones, cartilage and surrounding tissues, causing deformity.
The immune disturbance that underlies RA is thought to start in membranes beyond the joints. So why is it the joints that become so inflamed? Part of the answer seems to be that the blood vessels in the joints and in the synovial membrane are unusually porous, making it relatively easy for cells and molecules of the immune system to gain entry. Joint tissue is also rich in the citrullinated proteins targeted by autoantibodies in the most common form of RA.
Today’s strategy: “Treat to Target”
At one time, doctors did not treat RA aggressively, but aimed to ease pain using relatively mild drugs. By the early 2000s, evidence suggested that more aggressive treatment, enabled by a new generation of immune-modulating drugs, would reduce joint destruction. Current guidelines focus on a “treat to target” strategy: Aim for low or no disease activity, as assessed by such measures as counts of tender and swollen joints, patient reports about their pain and functioning, blood tests for substances indicative of inflammation, and joint imaging.
The current strategy relies on powerful drugs known as DMARDs (disease-modifying antirheumatic drugs), which can block inflammation and retard joint damage (see table). Patients begin a DMARD as soon after diagnosis as possible, and doctors adjust medicines promptly if the initial therapy does not work well. Physicians may also prescribe a corticosteroid to reduce inflammation.
The newest DMARDs, known as biologics, are substances produced by genetically engineered cells. Strong, they are also expensive. As biologics lose patent protection, manufacturers are developing chemically similar, less costly synthetic agents meant to act in the same ways. Five of these biosimilars have gained FDA approval, but litigation is so far preventing three from being used in the United States.
Even with many drug options, more than a third of patients do not achieve remission. So scientists are trying to characterize the various subtypes of the disease and exploring additional treatment strategies. A clinical trial announced on November 14 takes a new approach: It induces production of cells that specifically suppress the T cells and B cells involved in rheumatoid arthritis.
Hope for prevention
Several approaches are being explored for preventing RA in people at high risk of developing the disorder. Criteria for study participation differ but generally include having joint pain or producing autoantibodies, both in the absence of joint swelling.
–The PRAIRI Study delayed RA for about a year in patients who received a single dose of rituximab.
–The APIPPRA Study is testing weekly injections of abatacept.
–The StopRA Study is testing daily doses of hydroxychloroquine.
The mysterious case of RA in hand transplants
In August 2017, doctors at Johns Hopkins University School of Medicine reported in the Annals of Internal Medicine that RA developed in a 34-year-old woman a year after she received forearm and hand transplants. Her own hands were lost to a systemic bacterial infection. Only she, and not the donor, carried gene variants that increase risk for RA. The physicians suspect that the RA arose when her body reacted to unfamiliar proteins in the donor’s tissue.




