The rapid development of vaccines against Covid-19 is a scientific triumph. But the recipes for making these vaccines are the exclusive intellectual property of pharmaceutical companies, which means countries cannot manufacture an approved vaccine themselves, thus limiting distribution worldwide. For this and other reasons — such as problems with medical infrastructure and a lack of trained workers to administer the vaccine — most poor countries won’t be widely vaccinated until at least 2024.
Much of the process of discovering a new drug or vaccine — as researchers hunt for new candidates, and companies develop those into safe, effective products — is typically conducted behind closed doors. Even once a product is approved, patent protections prevent other manufacturers from making and selling it. Eventually, patents expire; but some aspects of the lifesaving science behind the development of those patented products — such as which candidates don’t work — often remain forever locked up in corporate silos, hindering research that may prevent future pandemics.
We believe there is a better, more equitable way of combating Covid-19 and any future pandemics: open science.
Open science operates under the principle that any scientific result should be shared immediately, without restrictions on its use. Some say open science won’t work for drug development, because it removes the financial incentives: Without the promise of patent protections, they believe, no one will assume the costs and risks to discover and develop new drugs. We disagree.
In March 2020, we started COVID Moonshot, which we believe is the first open-science effort to develop an antiviral drug. Now we are close to bringing an oral antiviral that’s effective against SARS-CoV-2 (the virus that causes Covid-19) to the clinic, with no patent protection. As soon as the drug is approved, any drug manufacturer around the world can manufacture and sell it without needing to license it, thus driving prices down.
This shouldn’t be a one-time thing: We believe open-science drug discovery against current and future pandemics is an essential public service — one that should be completely funded by governments for the benefit of their people.
The idea started with a tweet. Early in the pandemic, structural biologist Martin Walsh posted a 3D structure of one of the main proteins SARS-CoV-2 requires to replicate. We knew this protein was an attractive drug target because it doesn’t easily mutate, which can render a drug less effective. (In contrast, the coronavirus spike protein targeted by vaccines and therapeutic antibodies is more likely to acquire mutations.) The protein, called Mpro, is also present in other disease-causing coronaviruses, suggesting that any antiviral drug targeting Mpro has a good chance of being effective against future coronavirus pandemics.
Since time was of the essence, we decided to approach drug discovery in an entirely different way — by putting all our work online and asking the international scientific community to contribute. Ideas started streaming in right away, and scientists around the world have submitted more than 17,000 potential drug candidates targeting Mpro, each building on the ideas and data generated by others.
We have synthesized and tested more than 1,400 of these designs, using a network of collaborating labs including the Weizmann Institute of Science, the University of Oxford and Diamond Light Source. We also partnered with pharmaceutical companies (UCB, Boehringer Ingelheim), contract research organizations (Enamine, Sai Life Sciences), computing networks (Folding@home) and charities (DNDi and LifeArc) that donate labor and resources. This is a grassroots movement — our whole drug discovery process is based on crowdsourcing ideas, experiments and support from the scientific community.
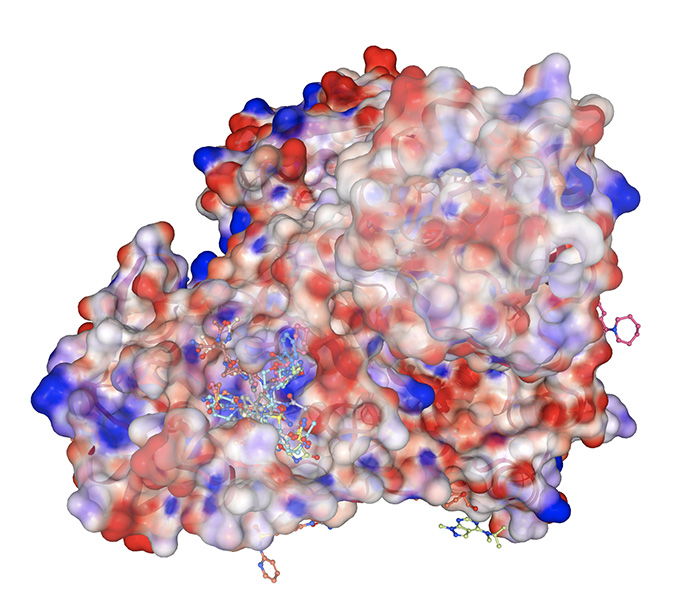
A variety of molecular fragments bind to Mpro, one of the proteins SARS-CoV-2 relies on to replicate. Designing a drug that blocks Mpro could stop the virus from reproducing.
CREDIT: ALPHA LEE
Now we have potential drug candidates that appear to do a good job of blocking the virus, as well as satisfying a variety of other properties that are required for a drug that can easily be taken as a pill (such as getting to and staying in the bloodstream). This, itself, is a remarkable achievement, which took slightly more than a year — a breakneck timeline in drug development. It typically takes several years to get to where we are now.
Even though multiple vaccines exist that help protect many people from contracting severe Covid, we still need oral antivirals that can cure people once they are infected. A shelf-stable pack of pills is much easier to distribute, store and administer worldwide than vaccines, needs to be used only by those who contract or are exposed to the virus, and has the potential to completely suppress viral loads to prevent symptoms and transmission.
We are currently working to identify funding partners to carry the effort into clinical trials in the next 12 months, and are looking to grants and charities to ensure that any approved medicine remains patent-free; we have recently secured a £8 million ($11 million) grant from the Wellcome Trust for preclinical regulatory studies. If successful, we believe this would be the first time in history that a clinical drug candidate has been crowdsourced from scratch.
During a global crisis such as a deadly pandemic, this is how drug development should work. Society appears to have collectively decided that governments should not financially support efforts to discover new drugs, even in matters of grave importance to public health. We buy our drugs from pharmaceutical companies, which assume the risks and costs of drug discovery, development and associated failures, and in return they charge the highest prices the market will bear.
With pandemics, this economic model doesn’t work. Our most advanced compounds appear to be equally effective against the virus that caused the SARS epidemic in 2003. In other words, if we had looked for an antiviral in 2004, we could have developed drugs capable of suppressing the Covid-19 pandemic more than a decade ago. But no private entity was willing to gamble that another SARS-related epidemic would occur within the lifetime of a patent. This market failure has already cost the world millions of lives and perhaps trillions of dollars.
We understand that drug development isn’t cheap — it cost an estimated $300 million to develop the antiviral Tamiflu, for example. (Tamiflu did, however, make nearly $1 billion in six months in 2009.) We estimate that we’ve spent approximately $1 million on supplies and other expenses so far, and that doesn’t include our donated time and resources (which likely exceed $1 million). We estimate we will need $50 million to $100 million to fund full clinical trials that carry our new antiviral all the way to regulatory approval, so it can be manufactured and distributed.
Open-science drug discovery contributes significantly to the public good. For example, as a by-product of our drug discovery effect, the COVID Moonshot has generated — and freely released — close to 50 percent of all available structural data about SARS-CoV-2 Mpro, and a wealth of biochemical data about Mpro inhibitors.
This provides a detailed atlas that helps drug hunters navigate this key protein in SARS-CoV-2 and related coronaviruses, shaving off years of wasted time in drug discovery efforts for future pandemics. It is a rising tide that lifts all boats. Many companies keep those data private to retain a competitive edge, so no one can learn from anyone else’s failures, dooming us to repeat each other’s mistakes.
Governments should be responsible for addressing existential threats to society. Unlike most illnesses, which are personal in nature, a pandemic cuts at the heart of civic life. Once an open-science drug is approved, it can be rapidly manufactured and sold cheaply around the world without legal barriers. Think how transformative that would be, and what a public service it would provide.
As scientists, we started the COVID Moonshot with the hope of doing our part to provide equitable global access to antivirals. Our progress shows that open-science drug discovery is scientifically viable. The ball is now in policymakers’ court to fund and take efforts like the Moonshot to patients. Until we start seeing infectious-disease drug discovery as a public service, pandemics will keep recurring, and no one will emerge unscathed.
This article is part of Reset: The Science of Crisis & Recovery, an ongoing Knowable Magazine series exploring how the world is navigating the coronavirus pandemic, its consequences and the way forward. Reset is supported by a grant from the Alfred P. Sloan Foundation.




