The global health community is working at unprecedented speed to find ways to treat and prevent Covid-19.
One of the largest efforts, a multinational clinical mega-trial called Solidarity, was launched by the World Health Organization to test several regimens: the antimalarial drug hydroxychloroquine; an experimental antiviral drug, remdesivir; and a combination of two anti-HIV drugs, lopinavir and ritonavir, with or without the immune-system modulator interferon-beta-1a. (On May 27, the WHO announced a temporary pause in testing the hydroxychloroquine regimen because of reports of adverse effects.)
Vaccine trials also are underway — at the time of publication, more than 170 candidates of various designs are in early testing, with most not yet ready for human trials.
The need is great, and so are the challenges. To learn about them, Knowable Magazine turned to Kathryn Edwards, a pediatrician and vaccine researcher at the Vanderbilt University Medical Center in Nashville, Tennessee. Edwards has decades of experience conducting clinical vaccine trials. She served on a National Academy of Medicine committee to review the response to the 2014–16 West African Ebola outbreak and recommend improvements, and is now on several advisory committees for clinical trials during the Covid-19 pandemic.
The coauthor of a 2020 overview of the ethics of clinical research during disease outbreaks in the Annual Review of Virology, Edwards discussed the complexity of conducting trials during such turbulent times. This conversation has been edited for length and clarity.
What issues arise around clinical trials during an outbreak? How do experts take ethical concerns into account?
It’s difficult to conduct clinical trials in the face of an epidemic, particularly during one with a newly discovered virus. You don’t know all about the disease at that point and you certainly don’t know how to treat it. You’re still learning. There is always a push to try and do something therapeutic: Often, there is a push to use medicines and other treatments that might be beneficial, but they haven’t yet been proven to be beneficial and they might even be harmful.
Although some might say that we should just empirically “try” a therapy, it is not the best approach. We should do careful studies on potentially therapeutic agents to determine if they improve the clinical outcomes, not make them worse.
What did you learn from the West African Ebola crisis and how did the situation differ from what we face today?
The Ebola outbreak was taking place in areas in West Africa with very weak medical infrastructures, so at the onset of the outbreak it was difficult to provide even supportive measures like intravenous fluids and antibiotics. There was a tension between the clinicians taking care of the patients and the researchers wanting to study different therapeutic agents. The mortality rate was so high that one of the issues was, “Why don’t we just give something to these patients, what is there to lose?”
The Covid-19 pandemic is slightly different. The mortality rate isn’t as high, fortunately, as it was in Ebola. Much of the outbreak currently is occurring in areas that have sophisticated medical systems and well-trained intensivists and pulmonologists who can provide supportive care.
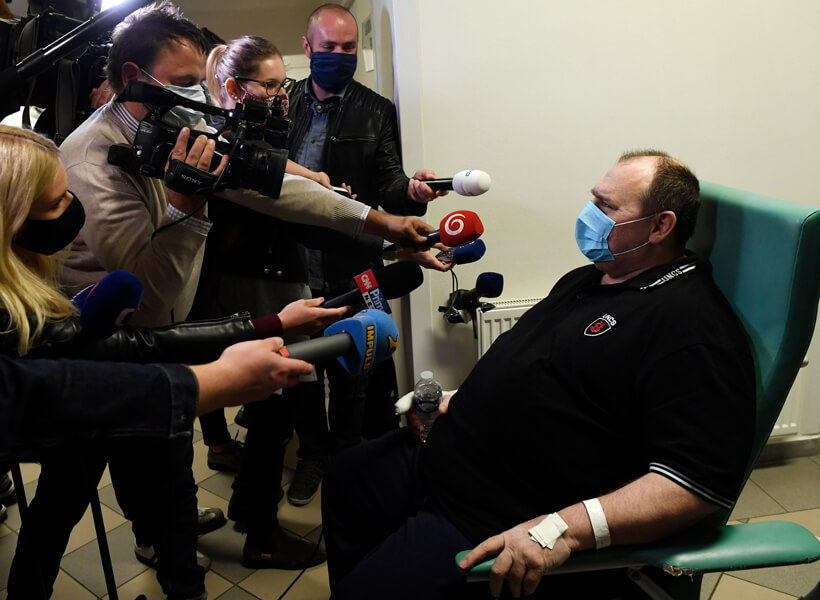
Covid-19 patient Robert Markovic is released to home care on May 5 after severe illness. Markovic, a 53-year-old taxi driver in the Czech Republic, was the first patient to be treated with the antiviral drug remdesivir in that country. The drug is one of several being tested in clinical trials.
CREDIT: CTK / ALAMY STOCK PHOTO
For Ebola, the lack of established infrastructures and systems, like investigational pharmacies [which supply drugs for clinical trials] and institutional review boards [which review clinical trials for ethical issues] made it difficult to do trials in a timely manner.
With Ebola, there also was a lot of community distrust and a lack of understanding, and communication with the local community was initially fragmentary. In contrast, with Covid-19 there has been extensive media coverage — that’s almost all that’s on the media — so people are much more comprehensively informed about the disease.
One of the frustrations during the West African Ebola outbreak was that by the time the therapeutic trials were set up the epidemic was waning, and there weren’t enough newly infected people to enroll in the studies to really gain meaningful answers. It was disappointing that the therapeutic studies that were done during the epidemic did not conclusively prove any drug to be beneficial. Small numbers of patients did receive various therapies and were compared to historical controls who did not receive therapy. But the management of patients improved with time, making historical controls less relevant.
So one of the lessons of the Ebola outbreak was to plan therapeutic trials carefully and to include contemporary controls so that you could actually assess the impact of the new therapy.
Are there other notable lessons from your work with Ebola?
I learned that it’s very important to engage the community in the research from the beginning — communicating with the community exactly what’s being studied, why it’s being studied, what this study will involve and what impact the study results will have. People need to understand what is happening.
And then, to make sure that the participating community will be able to access the studied drugs or vaccines in the future if they are proven to be effective.
One of the areas that was controversial during the West African Ebola outbreak was that the therapeutic and vaccine studies excluded pregnant women and children because they were deemed to be too vulnerable. Many felt like these groups were not given the opportunity to benefit, if there was benefit to be obtained. And the Ebola fatality rate in pregnant women was exceedingly high.
Experts have included pregnant women and children in the therapeutic and vaccine trials taking place during the Ebola outbreak that is occurring now in the Democratic Republic of the Congo. There has been an evolution in the understanding that these are vulnerable populations that shouldn’t be denied the ability to enroll in clinical trials. That’s been another thing that I think has changed, although for Covid-19 the impact on children has been much less than on adults.
There have been a number of initiatives and recommendations made, post-Ebola. The National Academy of Medicine report suggested that the WHO should take the lead in these initiatives. But they also suggested that other groups such as the Coalition for Epidemic Preparedness Innovations (CEPI) should begin to work on templates, protocols and standards for clinical trials for therapeutics and vaccines before the onset of the next pandemic.
Many treatments and vaccines are being tested against Covid-19. Here is a tally from the FasterCures initiative of the Milken Institute. The number changes often; these data are from May 25, 2020.
What are the potential challenges in a trial like Solidarity, the global trial of drugs for treating Covid-19?
A major challenge for therapeutic trials during a pandemic is determining and precisely recording the impact of the therapeutic agent on the patient. In enrolling patients in clinical trials, there is a randomization process: Some get the drug and some do not. The caregivers are blinded — they are not aware of which group receives the therapeutic and which one does not.
The problem comes when doctors use the agent outside of a clinical trial and don’t capture detailed information about the patient outcomes. There should be a very methodical and organized way to record patient variables such as the presence of virus, the titer of virus, measures of oxygenation and ventilator use, for example.
The clinical trials being conducted are measuring these outcomes. And given the number of Covid-19 patients that we have, therapeutic information can be rapidly gained on which therapies are beneficial and which are not. Some people who are not at academic medical centers may feel that they can’t capture the information in an organized way, but the Solidarity trial was organized so that many different clinical centers could participate.
Why do you think the WHO rolled out a therapeutic trial before turning to vaccines?
I think that was a wise choice. People are sick, there are therapeutic agents to be tested, and physicians and patients are both eager to find therapeutic answers. Vaccine trials are following closely.
Could you tell us a bit about Covid-19 vaccine trials?
Currently, there are 120 different vaccines listed in the WHO website in various stages of development, from preclinical to clinical. There are also a number of vaccine studies that have begun in Europe, China and the United States.
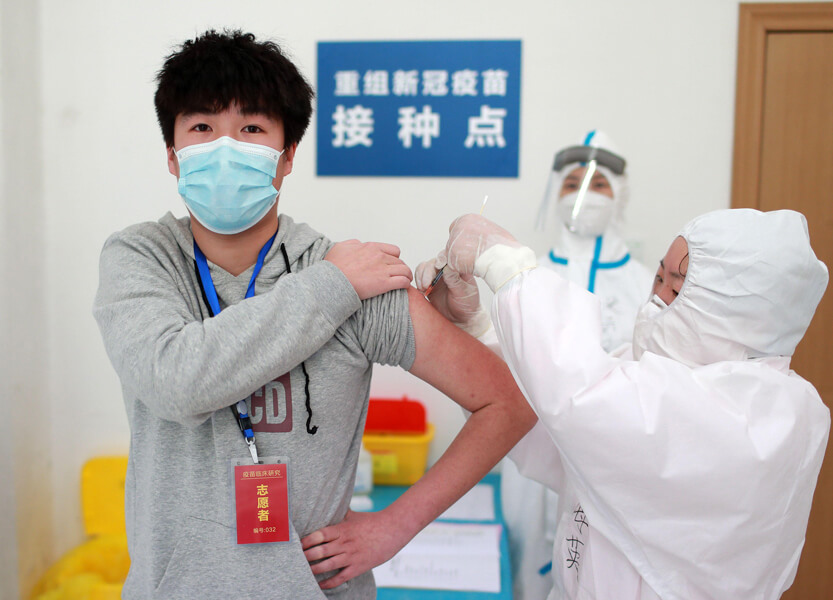
A volunteer in Wuhan, China, receives an experimental vaccine against Covid-19 on April 15. The man was one of 220 volunteers in Wuhan in a Phase 2 trial for this vaccine candidate. Scores of vaccines of many kinds are in development, and some are in early human trials.
CREDIT: SIPA USA / ALAMY STOCK PHOTO
The WHO has been very active in providing guidance and blueprints. Another thing that has happened since the West African Ebola outbreak has been the formation of CEPI. It is a non-governmental foundation that is funded by the Gates Foundation and also backed by the Wellcome Trust and by a number of nations who have made contributions.
CEPI is working on vaccines for diseases that have pandemic potential. It has been doing that for several years for Nipah virus and chikungunya and a number of other viruses. And they have also begun to rapidly look at vaccines for Covid-19.
What are the practical and ethical concerns for such human vaccine trials?
There should be preclinical studies, often starting in mice and then nonhuman primates, to make sure the vaccines will stimulate immunity and that they are safe in the animals. Then we move through successive Phase 1, 2 and 3 studies. The participants will need to be comprehensively informed about the risks of the vaccine and will need to commit to careful monitoring of reactions to the vaccines. The participants will also need to be followed carefully for adverse events that might occur in the year after vaccine administration.
As with therapeutic trials, the people who are enrolled in the studies, particularly the Phase 1 trials, need to understand what is being done and what are the potential risks and what are we going to do about mitigating the risk, because this is not a risk-free situation.
With regard to the Covid-19 pandemic, where are we heading?
Clinically, we are learning how to manage patients and how to predict which patients will get more severely ill. And hopefully, as we do our therapeutic trials, we will find new therapeutic agents.
Vaccine development is also on track. We’re hoping that within the period of a year to 18 months we’ll have a vaccine that we can give to people in the general population. That is a much accelerated timeline.
But we also need to learn more about the impact of various social distancing measures. The economic implications of Covid-19 are enormous: We can’t continue to live isolated with everything locked down forever. So we will need to gather information about how we can reopen society in the safest and most effective manner.
And we need to improve the robustness of our global health systems. The recent move by President Trump to withdraw US funding for the WHO is a real concern, as well. The WHO is a leader in providing guidance for low- and middle-income countries as they navigate the pandemic. This is a global pandemic. The WHO has an important role.
One thing that has been somewhat frustrating for me about the Covid-19 outbreak is that there has been reluctance by some politicians to listen to what the scientists are saying and to respect them for offering the best scientific information that is available. There should be a constructive and respectful dialogue between scientists and politicians.
Will we learn any lessons from the pandemic? Will our lives be different 10 years from now than they are currently? They will in some ways, but one of the things I have seen frequently in my long career is that people soon forget.
When I was a pediatric resident almost 50 years ago, I stood by the bed of a child who died of bacterial meningitis. And I couldn’t do anything. Now we have vaccines and this disease is gone. But now I hear people saying they don’t want their children to be vaccinated against meningitis because they no longer see the disease.
Well, diseases are an airplane ride away, as we know with Covid-19.




