Every one of the human body’s more than 30 trillion cells is a miniature powerhouse, able to glean its own energy from nutrients and synthesize molecules necessary to perform its duties, whether as a liver cell, heart muscle cell or neuron. Whatever a cell doesn’t need or can’t use gets recycled by tiny sacs of enzymes called lysosomes — from the Greek for “digestive body” — which take care of cellular waste disposal.
Mammalian cells are studded with hundreds of lysosomes, which contain more than 60 different enzymes that dismantle worn-out cell parts and bacteria, and recycle a cell’s own fats, sugars and proteins. These enzymes are active only in the highly acidic environment found inside lysosomes, an important safeguard. (Otherwise, if a lysosome were to leak or burst, the enzymes could kill everything in the cell.) When a lysosome comes across cellular debris it can’t reuse, it fuses with the cell membrane and dumps the waste out of the cell in a process called exocytosis. In the last decade, biologists have expanded their view of the lysosome, showing its central role in cellular health and disease.
The complex dance of destruction, renewal and removal doesn’t always run smoothly. When an enzyme is missing due to a genetic mutation, or another lysosomal error occurs, waste can build up in the cell and cause devastating diseases. Many genetic lysosomal disorders such as Tay-Sachs and Batten disease begin early in life and lead to blindness and premature death, but mounting evidence suggests that faulty lysosomes can also contribute to diseases that arise later, such as Alzheimer’s and Parkinson’s.
The few current therapies that exist for lysosomal disorders, such as those that replace missing lysosomal enzymes, merely slow the diseases’ progress. Gene therapies that boost lysosomal function hold promise, but human trials are still in preliminary stages. Another potential lead for new therapies comes from the discovery of a “master” protein that regulates lysosomes, called transcription factor EB, or TFEB.
Baylor College of Medicine geneticist Marco Sardiello helped to discover TFEB’s role and recently coauthored a review in the Annual Review of Neuroscience about lysosomes’ role in brain health. In November, he and his team reported in Nature Cell Biology that disrupted transport of lysosomal enzymes within the cell underlies Northern epilepsy, a form of Batten disease also known as neuronal ceroid lipofuscinosis 8. Knowable interviewed Sardiello about why the brain is particularly vulnerable to lysosomal glitches, and how TFEB-based therapies could help.
This conversation has been edited for length and clarity.
What is a lysosome, and what role does it play in cells?
Think of the cell like a big town, a big city like Houston. The nucleus is like the administrative part of the city, the downtown. It contains the DNA, all the instructions for how to run the cell, similar to the administrative offices that have instructions for how to run a city. Then there are the energy plants, which are the mitochondria — they provide the energy for the cell.
Just like in a city, the natural activities of a cell produce waste that has to be eliminated, or even better, recycled. Lysosomes collect lipids, DNA, RNA, sugars, proteins — you name it, any kind of molecule that has exhausted its function — and break them into tiny parts that the cell can use again.
What goes wrong in lysosomal disorders?
There are more than 50 lysosomal storage disorders, most of which arise from mutations in the genes coding for lysosomal proteins. In any normal segment of the population, about one in 5,000 people is affected.
In many of these disorders, one of the lysosomal enzymes is not working properly or is completely absent, so there is an accumulation of waste material that was supposed to be degraded but is not. My lab focuses on Batten disease, a lysosomal storage disorder which is one of the most frequent neurodegenerative diseases of childhood.
The development of children with some forms of Batten disease appears quite normal, so parents don’t suspect that anything is wrong at first. But by the age of four or five, parents start to notice that their kids have to put a piece of paper really close to their eyes in order to read. The doctor sees that there is retinal degeneration, and at this point there are many possible options — many diseases that could lead to the degeneration of the retina — so it often takes a while, even years, to identify the gene that is mutated.
After retinal degeneration, the degeneration extends to the whole brain. Little by little, the kids start to lose function and they become completely blind. Then, all upper-level functions start to go down, like language and cognitive ability. They lose the ability to walk or to feed themselves and by the second decade of life they can become bedridden. Eventually there is premature death, usually by the beginning of the third decade.
Within a cell, lysosomes help with recycling and waste removal through a number of pathways. Rich in powerful enzymes that can break down molecules and even entire organelles and bacteria, lysosomes fuse with sacs carrying cellular debris (via autophagy) or pathogens from outside the cell (via phagocytosis). The enzymes chop up the contents into recyclable bits, such as single amino acids, which are then released into the cell for reuse. Any undigested material is shuttled outside of the cell through exocytosis.
What treatments currently exist for lysosomal disorders?
The classical approach for lysosomal disorders is called enzyme substitution therapy, which means that you provide a missing enzyme through an injection into the bloodstream. This approach is quite effective for organs like the liver and the muscles, which are also affected in lysosomal disorders. The problem is that when you inject something in the bloodstream, it is very difficult to get it into the brain because of the blood-brain barrier. There are some clinical trials in which doctors are directly injecting these enzymes into the brain or the cerebrospinal fluid, and so far those are going well.
But for some lysosomal disorders, what is missing is not an enzyme. For Batten disease subtypes 3 and 7, for example, what is missing is a protein in the lysosome membrane itself. For Batten disease 6 and 8, the missing proteins sit in membranes of a cell organelle called the endoplasmic reticulum. That is something you cannot replace with enzyme substitution therapy, because these proteins cannot travel from one cell to another.
The function of these membrane proteins is not completely understood, but my laboratory recently reported the discovery that the CLN8 protein, which is missing in Batten disease subtype 8, works as a transporter for newly synthesized lysosomal enzymes. When genetic mutations affect the production of CLN8, the trafficking of lysosomal enzymes is impaired and fewer enzymes make it to the lysosome, thereby decreasing the lysosome’s ability to dismantle the cellular material that it receives.
For lysosomal disorders that involve a missing or dysfunctional membrane protein, there’s really not much one can do beyond palliative therapy. This is why we so desperately need something that can slow down or halt the progression of the disease — at least until gene therapy becomes a real clinical option, which it isn’t yet.
What therapeutic approaches are you and your colleagues exploring?
In 2009, my colleagues and I identified transcription factor EB, or TFEB, as the master protein responsible for the coordination of the expression of lysosomal genes. That led us to a very useful concept — to have more lysosomes in a cell, you just have to add more TFEB. TFEB is expressed in the cell, but it’s mostly inactive, waiting for a signal to start the transcription of genes that contain the instructions to make more lysosomes. We have found ways to use drugs to activate TFEB and to get the cell to make more lysosomes in neurons and other cell types.
So far, we have done pre-clinical trials with TFEB in two different mouse models of lysosomal disorders, including Batten disease. In both mouse studies, the therapy led to a longer life span, reduced buildup of waste in brain cells, and lowered signs of neuroinflammation and neurodegeneration. It is possible that we will see the first attempt to translate this into human therapy as early as 2019, which makes us very happy.
What about the limits of the mouse models? How similar is the disease in mice and people?
Our brains are very similar to that of the mouse, biologically. The real problem is the necessity to scale up — to make sure that the doses used in the mouse can be scaled up to the human brain. The brain of the mouse is really small compared to ours, so whenever you’re delivering a drug — whether it’s a classic drug or gene therapy — you need to make sure that the whole human brain is getting the drug or it’s not going to work.
What role do lysosomes play in diseases like Alzheimer’s and Parkinson’s, where waste also builds up in cells?
What is emerging now is that there is also a huge involvement of the lysosome in neurodegenerative diseases such as Alzheimer’s and Parkinson’s. In Parkinson’s disease, there is accumulation of a protein called alpha synuclein, and in Alzheimer’s disease we have the accumulation of hyperphosphorylated tau and beta-amyloid proteins.
In familial forms of both diseases, we find mutations in lysosomal genes that participate in the collection of the waste material, in activation of lysosomal enzymes or in recycling and transport.
It is very likely that the lysosomal defect in neurodegenerative diseases such as Alzheimer’s is more subtle than in genetic lysosomal disorders, so it takes way longer to develop the accumulation of this waste material. The cell can take a lot of damage before giving up. Just like a city — in Houston we had Hurricane Harvey a year ago, but the city has been able to recover.
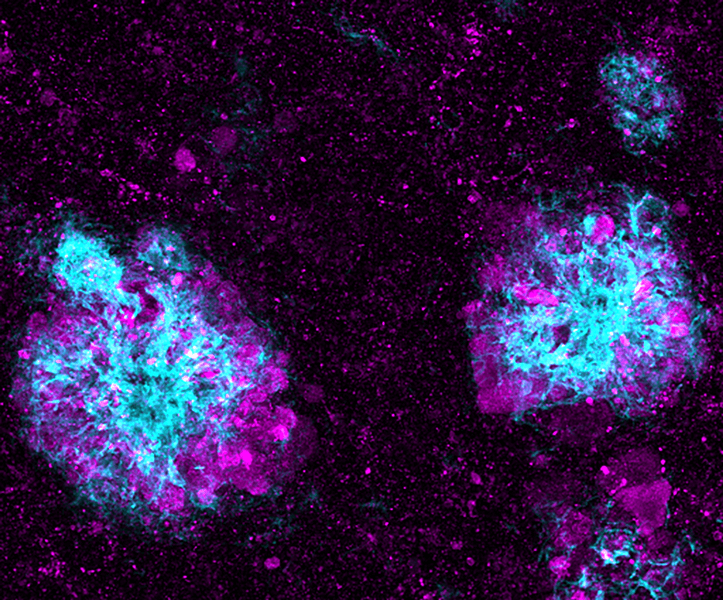
In a mouse model of Alzheimer’s disease, lysosomes (shown in purple) pile up in the nerve cells that surround amyloid plaques (shown in blue). Problems with lysosomes’ ability to process or remove debris in brain cells have been found in people with familial forms of Alzheimer’s.
CREDIT: SWETHA GOWRISHANKAR AND SHAWN FERGUSON / YALE SCHOOL OF MEDICINE
Why do dysfunctional lysosomes take such a toll on the brain?
There is still no straightforward answer, or at least not a simple one. It looks like there are many different reasons why this is happening. One of the reasons could be that neurons are post-mitotic, meaning that they don’t divide. A neuron is forever — so if there is a problem with the lysosome and a neuron is damaged and dies, it cannot be replaced. If this happens in the muscle, it can always be replaced by another cell.
Another part of the answer could be that when most types of body cells duplicate — when one cell becomes two — they also dilute their wastes. But, again, this does not apply to neurons because they do not divide. So they just keep accumulating waste material — they don’t know how to get rid of it.
Neurons do perform exocytosis, but this may not be enough to compensate for the loss of recycling inside the cell. One thing that TFEB does is accelerate the rate of lysosomal exocytosis. If TFEB is active, or there is more TFEB in the neurons, the neurons perform more lysosomal exocytosis and are therefore better at getting rid of the waste they can't degrade. This is an important aspect of therapies based on TFEB — TFEB enhances the capability of the cell to clear itself by simultaneously increasing lysosomal power and lysosomal exocytosis.
Given how many different things can go wrong with lysosomes, do you find it impressive that things work so well most of the time?
Honestly, this surprises me every single day. I mean, the more we study the cell, the more amazing I find it that we are even alive.




