Pig organs in people: The future of cross-species transplants
Can genetically modified animals help ease the shortage of organs? After years of research into xenotransplantation, the field is at a turning point — yet risks and ethical issues remain.
Support sound science and smart stories
Help us make scientific knowledge accessible to all
Donate today
More than 100,000 people in the United States are waiting for a new heart, kidney or some other organ. Many will die waiting. Some scientists see new hope for these people in organs from pigs that have been engineered to work within the human body.
Such species-to-species transplants, called xenotransplantation, offer a technical solution to a basic problem: There are more people in need than there are organs, be they from living or brain-dead donors, to go around.
“Unfortunately, as we speak, someone is dying just waiting for an organ,” says surgeon Muhammad Mansoor Mohiuddin, director of the cardiac xenotransplantation program at the University of Maryland in Baltimore.
Over the past few years, a handful of people in the United States and China have received specially modified pig kidneys, hearts and livers, but getting those organs to function safely in a person is a huge challenge, as laid out in the 2024 Annual Review of Animal Biosciences. Now, thanks to technological and medical advances, United Therapeutics, in Silver Spring, Maryland, is starting the first official clinical trials of xenotransplantation, and many researchers believe the procedure could eventually become routine.
Yet there are ongoing questions, including risks that pig organs will transmit viruses to people, and a number of ethical concerns. Here’s a look at the state of play and what may lie ahead.
Fighting rejection
After a long history of animal transplant experiments, scientists have zeroed in on pigs, or minipigs, as an organ source. The animals breed and grow quickly, their organs are about the right size, and there aren’t many pathogens that infect both pigs and people.
“I think a pig is almost an ideal donor for human transplantation,” says Wenning Qin, senior vice president for innovation at eGenesis, a Cambridge, Massachusetts-based company that engineers minipigs as a source for organs.
But without any special tweaks to those organs, the human body will immediately attack them. This “hyperacute rejection” is kicked off when human antibodies recognize as foreign three sugar molecules on the blood vessels of a pig organ. The antibodies stick to the cells and set off a chain of events that clots blood, blocking its flow. “Within 10 minutes, the organ would turn from the color of pink to black,” says Qin. “The organ is dead.”
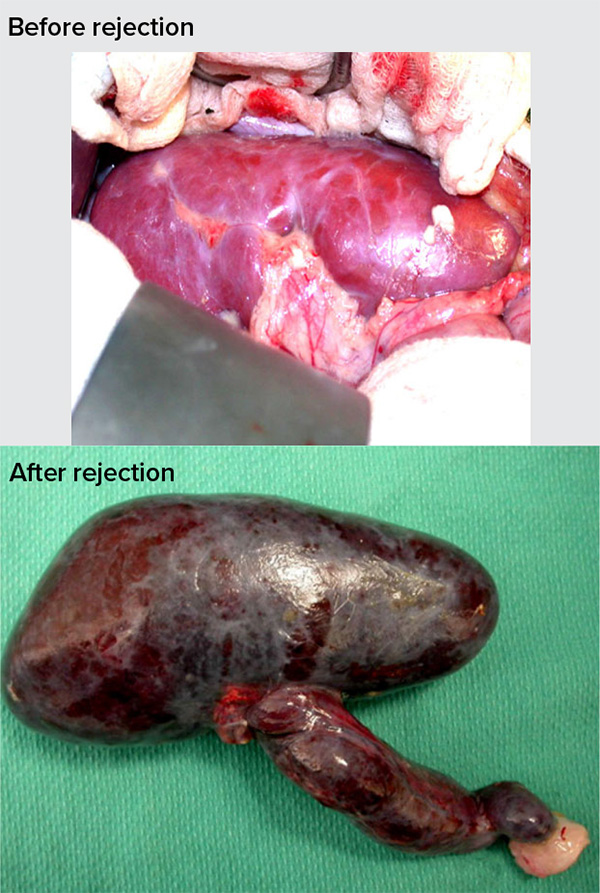
In a process called hyperacute rejection, the recipient’s antibodies recognize an organ as foreign and attack it immediately: Blood flow becomes blocked, quickly killing the organ. The pig organs used for transplantation have been engineered to avoid such rejection.
CREDIT: D.K.C. COOPER ET AL / XENOTRANSPLANTATION 2016
To solve the problem of hyperacute rejection, companies such as eGenesis and United Therapeutics have used the CRISPR/Cas-based gene editing system to modify the DNA of pigs. For example, eGenesis destroyed the three genes responsible for those problem pig sugars. But the human immune system still has ways to recognize and reject the foreign organ — just later on. And standard immunosuppressant drugs, designed for human-to-human transplants, can’t fully prevent that response to xenotransplantation, says Mohiuddin.
To help address this later-stage rejection, both companies also added several human genes to the pig cells. These genes make proteins that sit on the cell surface, disguising the pig cells as human. In 2023, eGenesis reported that five out of 15 cynomolgus monkeys who received kidneys from the company’s pigs survived for more than a year.
And to further quell that later-stage response, Mohiuddin and others have developed medicines that suppress immune cell responses further. In experiments with pig hearts transplanted into the abdomens of five baboons, this treatment allowed the transplanted hearts to survive for up to 2½ years.
Other researchers hope to eliminate the need for long-term drugs by training the immune system of the organ recipient to ignore transplanted tissues, be they human or otherwise. Megan Sykes, director of the Columbia Center for Translational Immunology in New York City, is tackling this by transplanting additional tissue from the donor’s immune system — either bone marrow cells or the thymus — into the recipient. This approach, it’s hoped, will make a recipient’s immune system more tolerant of donor tissues. She’s currently testing whether she can safely withdraw immunosuppressant drugs from baboons that received pig organs plus thymuses. (Sykes has received research funding from United Therapeutics.)
Sykes says that “it’s an exciting time” for the field of xenotransplantation. “I think we’re going to see some significant progress in patients in the near future.”
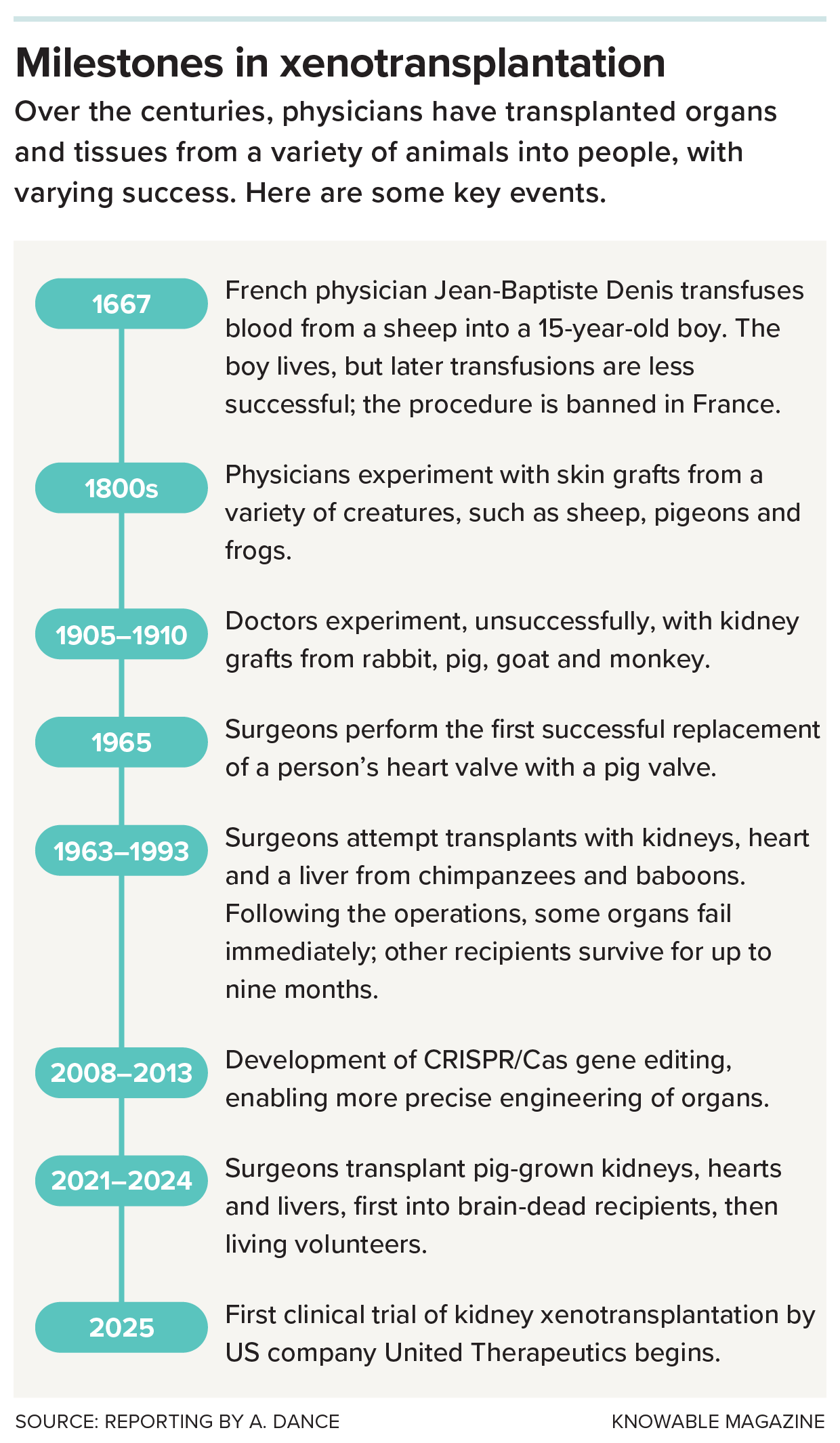
Here are some key moments in the history of cross-species transplantation.
Ethical concerns
In human beings, more than a dozen pig-organ transplants have occurred so far. The first transplants were short-term experiments in patients who were brain-dead, so there was no further risk to human health. In a handful of these cases, researchers reported that genetically edited pig hearts could beat, livers made bile, and kidneys were able to function, making urine, without immediate rejection.
From there, surgeons moved on to live organ recipients. These volunteers have been too ill or were otherwise ineligible for a human organ transplant.
Some ethicists have raised concerns over how these human patients are being selected. In these early transplants, physicians approached eligible recipients and applied for approval from the Food and Drug Administration under the “compassionate use” pathway that allows a seriously ill person to receive an unapproved or untested therapeutic.
“These are patients who are being given an offer they can’t refuse,” says L. Syd Johnson, a bioethicist at SUNY Upstate Medical University in Syracuse. They may hope for a lot more than they’re being promised, Johnson adds: “I don’t think people are undergoing major surgery and immunosuppression and all of the various risks of those things to survive for three days or three weeks or six weeks.”
Mohiuddin, who says the research community is “totally indebted” to these patients, adds that the recipients are well aware of the risks of these experimental procedures. The first four people to receive pig kidneys or hearts did die within weeks or months, but doctors and scientists learned valuable lessons, he says — for example, that it’s important to start immunosuppression before the transplant takes place.
Such findings helped later transplants go better; an Alabama woman, who received a pig kidney at the New York University Langone Transplant Institute in November 2024, was cleared to head home from New York in late February.
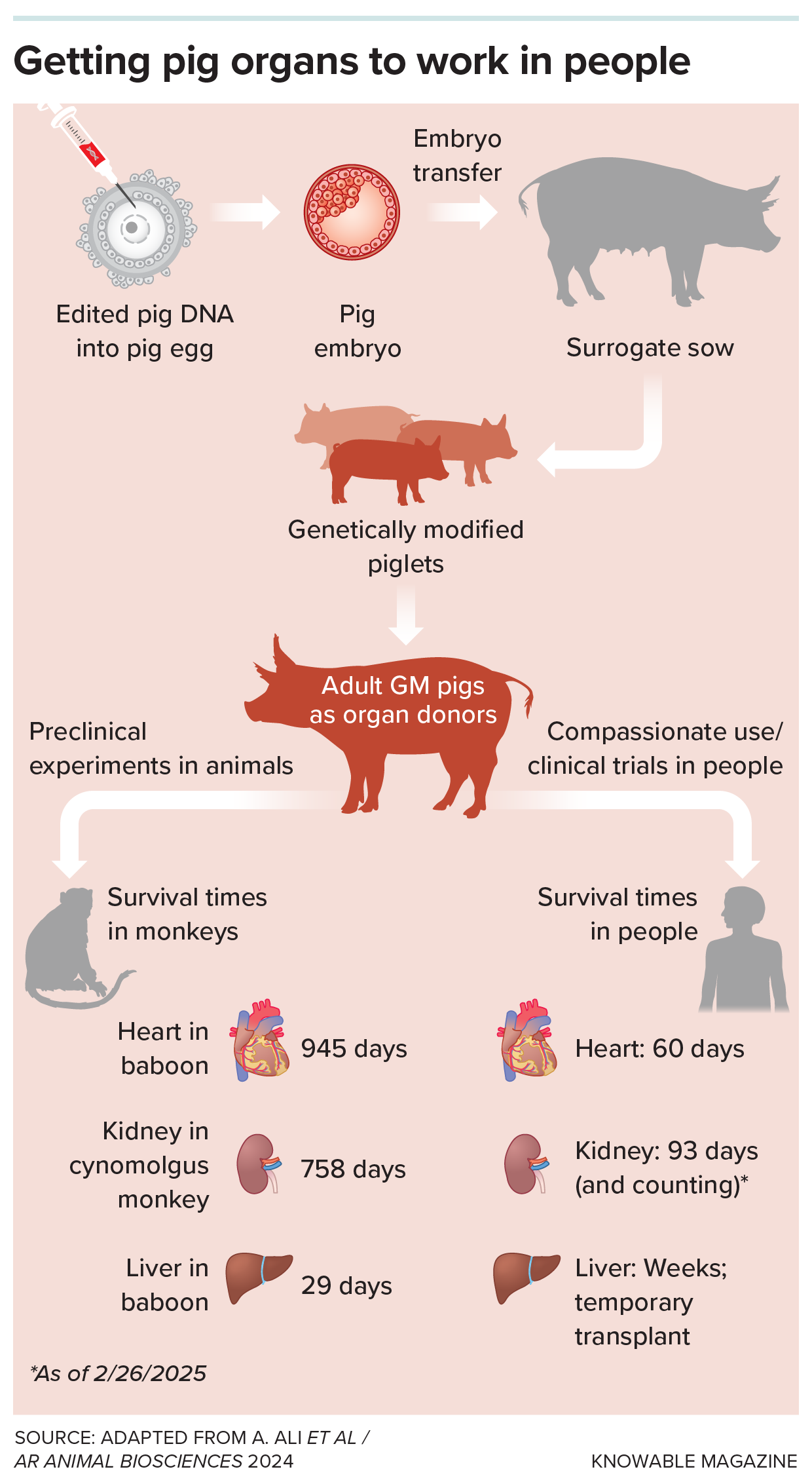
To produce organs that the human body won’t reject, scientists edit pig DNA, transfer it into an egg, and grow modified piglets. Such organs have lasted for weeks to years in experimental transplants into monkeys and, thus far, for months in people.
The virus spillover concern
Scientists also have concerns about pigs carrying viruses that might infect human transplant recipients, or even people those patients interact with. Of particular concern are ones called porcine endogenous retroviruses that hide out in the pig genome. Again, CRISPR/Cas gene editing provided a solution: eGenesis made additional edits to its animals; 59 out of the 69 changes were to destroy these retroviruses.
A different pig virus did show up in one transplant recipient, David Bennett: He was the first to receive a United Therapeutics pig heart, with 10 genetic modifications, in 2022. Tests after the transplant found some DNA from a pig cytomegalovirus in his blood. The virus was probably inactive, and did not infect any of Bennett’s own cells, says Mohiuddin, who co-led the transplant team. Bennett died of heart failure, which Mohiuddin says was likely a result of rejection, after about two months. More sensitive virus testing is now available, Mohiuddin adds.
Even a small risk of viral transmission has broad implications for people, because serious and even pandemic viruses such as HIV, and probably Covid-19, have resulted when viruses from other animals landed in humans. Viruses that cross from one species to another can evolve to become nastier, especially in people with weakened immune systems — like sick people taking immunosuppressants.
Thus, the US Public Health Service and the International Xenotransplantation Association have recommended lifelong infection monitoring in xenotransplant recipients. But that means patients don’t have the usual right of a trial participant to withdraw from a study at any time, Johnson notes. Monitoring might extend to people they interact with, who never consented to a study at all.
Another protection against viruses is to keep the pigs in sterile environments — but the animals may not be so keen on that. Pigs are intelligent, social animals, and they really do like to wallow in mud; sterile lab conditions are likely to cause them “psychological and social harm,” Johnson says. Though the world slaughters nearly 1.5 billion pigs every year for food, she says it doesn’t necessarily follow that it’s ethical to create additional animals for medical use.
Who benefits, and who pays?
If xenotransplants become standard medicine, it raises another ethical quagmire: How will these organs be distributed, and who will pay for them? The first two transplants at the University of Maryland cost about $1.5 million each, Mohiuddin says; one was covered by the university and the other by the study sponsor, United Therapeutics.
“I would much rather see us putting our time and resources into other things, into preventing organ failure, into treating organ failure, into organ regeneration,” Johnson says.
Xenotransplant proponents, however, are eager to commence clinical trials. The FDA has authorized eGenesis and collaborators to conduct three additional compassionate use kidney transplants. The first operation, in a New Hampshire man, took place on Jan. 25 and the patient has been discharged from the hospital. United Therapeutics, meanwhile, has gotten the FDA go-ahead for a clinical trial in kidney patients, starting mid-2025 with six patients and potentially going up to 50 recipients.
“We are not claiming that we have a perfect solution yet,” says Mohiuddin. But he hopes that, eventually, xenotransplants can become a valid supplement to the human organ supply. Additional edits to pig organs or progress in tolerance medicine might make long-term immunosuppression unnecessary.
“You’ll have no organ shortage, if you get to that stage,” Mohiuddin says.
10.1146/knowable-022625-1
TAKE A DEEPER DIVE | Explore Related Scholarly Articles