Targeting the racial disparity in kidney disease
Some people of West African descent face a higher risk of renal failure. New drugs based on gene research may help right the ship — if they can reach everyone who needs them.
Support sound science and smart stories
Help us make scientific knowledge accessible to all
Donate today
The result was exciting. Thirteen people with serious kidney disease given a new type of drug for three months had, on average, seen the amount of protein pathologically leaking into their urine fall by almost 50 percent. In this elemental test of kidney function, some participants saw an improvement of more than 70 percent.
But other things make this clinical trial, published in March 2023, stand out further.
The first is the rapidity with which a scientific discovery led to a targeted therapy. The drug — called inaxaplin — targets a protein called APOL1, which was first implicated in kidney disease just 14 years ago. And the company behind the drug, Boston-based Vertex Pharmaceuticals, only began working on APOL1 in 2015.
The second is that the results suggest a way to address one of the starkest racial health disparities in the United States: the rate of kidney failure in Black Americans, more than three times higher than that for those of European ancestry.
Every volunteer in Vertex’s study was African American. But the reason they were recruited was because of which versions they had of the gene carrying instructions for making APOL1. Certain APOL1 gene variants — called G1 and G2 — are linked to renal failure. Trial participants carried either two G1s, two G2s, or one of each.
These gene variants were discovered in two United States studies in 2010 — exclusively in Black individuals. Carrying just one of the variants has essentially no consequences for kidney health, but possessing two gives an estimated 15 to 20 percent lifetime risk of experiencing kidney disease.
And the variants are jarringly common. The proportion of Black Americans who have two of them is estimated at around 13 percent.
“If you were to look in the literature for genetic variants that are that common and that powerful,” says David Friedman, a nephrologist at Harvard University who codiscovered the variants, “you would have a hard time finding more than maybe two or three in the same general weight class.”
A short animated video from Vertex Pharmaceuticals explains the company’s approach to studying and treating APOL1-mediated kidney disease.
CREDIT: VERTEX PHARMACEUTICALS
Friedman and his Harvard colleague, kidney disease researcher Martin Pollak, propose that the reason for that high frequency is rooted in history. They found that having just one copy of a G1 or G2 variant protects against a deadly form of African sleeping sickness.
The global distribution of these APOL1 variants indicates that they probably originated and quickly spread in West Africa, where sleeping sickness is endemic. Because most Black Americans are descended from West Africans brought to the United States as slaves, the variants remain prevalent among Black people in North America.
These discoveries have reframed a disparity that is palpable in the everyday practice of nephrologists. Previously, the fact that more than 35 percent of Americans with kidney failure are Black, even though Black Americans are only around 12 percent of the population, was often attributed to socioeconomic factors, says Marva Moxey-Mims, a pediatric nephrologist at the Children’s National Hospital in Washington, DC. Those factors do play a significant role, she says. “But to have hard scientific data to say, ‘There’s a biologic reason this group of people may develop chronic kidney disease at a much higher rate than others’ — I think it was almost like a sigh of relief, that there’s something else.”
Moxey-Mims and her colleagues now hope to use APOL1 screening to transform policies around kidney transplantation. And with Vertex currently testing inaxaplin in a large, placebo-controlled trial, hopes are high that medical options will soon expand for the tens of millions of people globally whose APOL1 genes put them at risk of kidney failure.
“If you were to look in the literature for genetic variants that are that common and that powerful, you would have a hard time finding more than maybe two or three in the same general weight class.”
— DAVID FRIEDMAN
Evolution’s side effects
Kidney disease has many causes. It results most commonly from diabetes or hypertension, but sometimes from infectious diseases like HIV, or from lupus, an autoimmune disease. Occasionally, it occurs in isolation, such as in a disease called focal segmental glomerulosclerosis. FSGS often strikes children or younger adults, causing kidneys to degenerate with no other disease signs. The volunteers in 2023’s small inaxaplin trial all had FSGS, and it has been central to uncovering APOL1’s importance.
The United States’s stark racial disparity, whereby end-stage kidney disease affects approximately 7.5 percent of African Americans, compared to roughly 2 percent of Americans of European descent, has been clearly documented for decades. By the 2000s, researchers suspected that genetics contributed. They noted that renal failure often runs in African American families. And studies controlling for factors such as poverty, poor healthcare access, diabetes and hypertension couldn’t fully account for the disparity.
In 2008, geneticists showed that an area of human chromosome 22 tracked with FSGS and with cases of non-diabetic kidney failure in African Americans. Then in 2010, two research groups, one of them Pollak and Friedman’s, established that the G1 and G2 variants of APOL1 were responsible.
At that time, APOL1 was alien to kidney biologists. But it was known to parasitologists who study single-celled protozoa called trypanosomes — some of which cause African sleeping sickness.
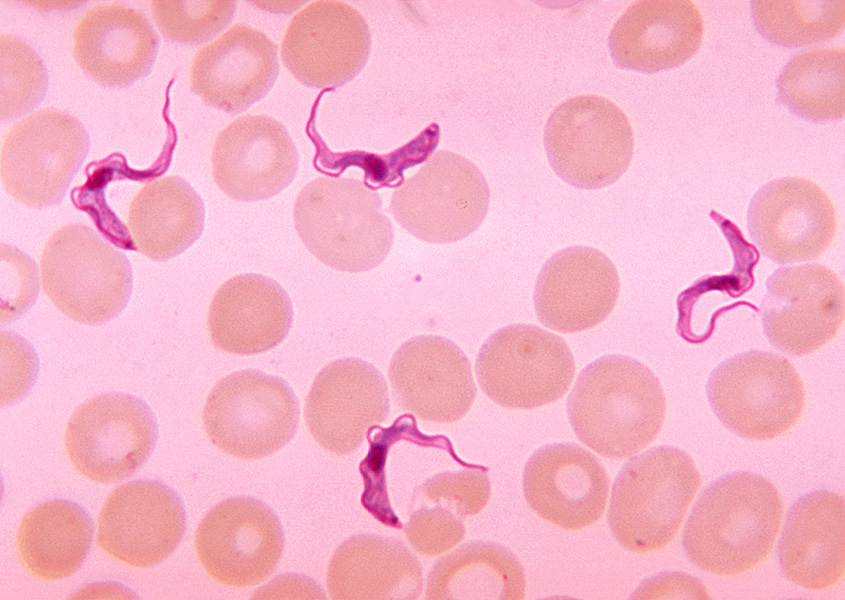
Trypanosome parasites (purple) shown next to human blood cells. These single-celled parasites cause African sleeping sickness. People with certain variants of the APOL1 gene are protected against sleeping sickness but are at higher risk for kidney disease.
CREDIT: CDC / DR. MYRON G. SCHULTZ
Researchers had previously shown that APOL1 proteins circulating in the blood cause natural immunity against one trypanosome species, although not against the trypanosomes that cause sleeping sickness. Friedman and Pollak showed that both G1 and G2 variants could kill a species of trypanosome that causes sleeping sickness. And, crucially, a person needs only one dose of G1 or G2 to be protected.
With surveys showing that G1 and G2 account for around half of all APOL1 genes in Ghana and Nigeria, the current hypothesis is that roughly 5,000 to 10,000 years ago, the mutations leading to G1 and G2 occurred in West Africa — and spread rapidly in the region, though not so much elsewhere in the African continent, because they conferred immunity to sleeping sickness.
The classic example of this type of evolution is sickle cell disease. In that case, having one disease-related version of a gene (for the blood protein beta-hemoglobin) protects against malaria, but having two causes sickle cell disease. As the price for overall protection against a lethal parasite, a fraction of a population gets two sickle cell variants and develops serious disease.
APOL1, sleeping sickness and kidney disease seem to have a similar evolutionary pattern.
An enigmatic gene
Kidney biologists are still figuring out how the G1 and G2 variants of APOL1 change kidney function. The gene itself exists only in a handful of primate species; kidneys of chimpanzees and the rodents that medical researchers often work with function perfectly well without APOL1. Parasitologists once even discovered a man with no functional APOL1 genes whose kidneys seemed just fine.
Today, researchers agree that the APOL1 protein allows ions to pass through biological membranes, and that the G1 and G2 versions are overactive or have some kind of altered, toxic activity. Various scientists hypothesize that this disrupts the membranes of kidney cells or compromises some internal cell component. “I don’t think anyone can say with confidence that they know the mechanism,” says Friedman, who wrote an overview of the APOL1 gene and kidney disease with Pollak in the 2020 issue of the Annual Review of Physiology.
Interestingly, having two copies of G1 or G2 increases the risk of developing multiple types of kidney disease. The chance of FSGS increases about 17-fold, and that of hypertension-induced kidney disease sevenfold to 11-fold. The risk of several other kidney disorders is moderately raised, though the variants don’t seem to affect the risk for diabetes-related kidney disease. Strikingly, people carrying two risk variants who contract HIV are tens of times more likely to develop kidney disease.
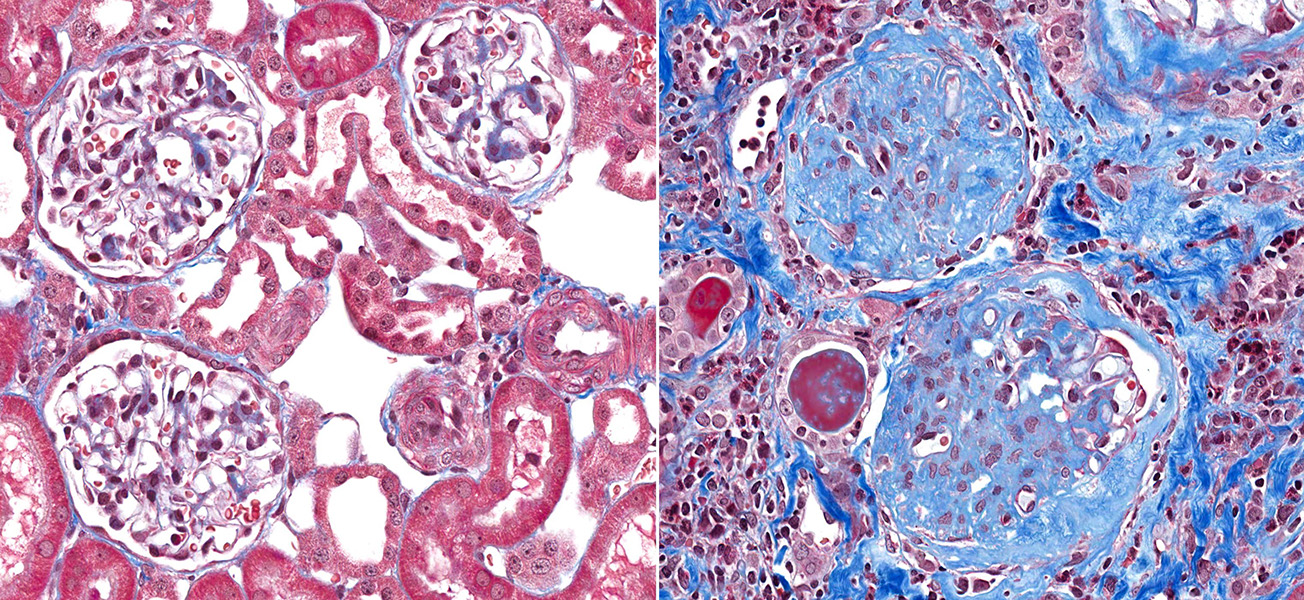
A microscopic view of healthy kidney tissue (left) next to one of kidney tissue displaying glomerulosclerosis (right). The blue dye shows the accumulation of scar tissue within the kidney’s tiny blood-filtering vessels, the glomeruli; this stops the kidney from functioning.
CREDIT: VETPATHOLOGIST / SHUTTERSTOCK
But despite raised risks, most people with a high-risk genotype do not develop kidney disease. Moxey-Mimms explains it this way: The genes probably make a person vulnerable to other risk factors such as hypertension, certain viral infections or other environmental insults. “It’s a second, or maybe even third, hit that causes you to develop kidney disease,” she says.
Nevertheless, APOL1’s critical involvement means that no matter what form of kidney disease a person has, if they have the high-risk genotype they are now classified as having APOL1-mediated kidney disease. And nephrologists think that drugs targeting APOL1 will treat or prevent sickness in such people.
New medicines
To researchers, the new functions that G1 and G2 introduce immediately implied that therapies should aim to reduce the APOL1 protein’s function. Odds for safety were good, scientists reasoned, since APOL1 appears dispensable for routine kidney function. “Here we have a gene that, if it didn’t exist, you’d be fine,” says Pollak. “So you can inactivate APOL1 — you can inactivate it totally.”
Several companies are pursuing APOL1 programs. AstraZeneca has partnered with California-based Ionis to develop a genetic therapy to reduce the production of APOL1 protein. In May, the companies presented a small (Phase 1) safety trial in which blood levels of APOL1 protein were reduced in volunteers without serious side effects. An AstraZeneca spokesperson says the companies are deciding whether to begin a Phase 2 trial to test the treatment’s effects on disease.
Vertex’s approach of creating a small molecule that blocks the APOL1 protein is also being taken by Maze Therapeutics, a small biotech company in South San Francisco. In December 2023, Maze announced that it was commencing safety trials in people.
But Vertex’s placebo-controlled Phase 2/3 trial of more than 400 people is the current focal point. Unlike the earlier FSGS-focused trial, this study — scheduled to finish in 2026 — is investigating multiple kidney disease types in people with two APOL1 gene variants, including children as young as 10 whose kidneys are failing.
Ogo Egbuna, Vertex’s vice president of clinical development, anticipates that participants will receive the drug for around two years. The study will assess several markers of kidney health and how long it takes for people to progress to dialysis, transplant or death.
Pollak, who with Friedman has consulted for Vertex, is optimistic for the field. “I’m convinced that the general approach that some of these companies are taking is going to be effective,” he says.
Transplant implications
Insights into APOL1 may transform nephrology in another way — potentially reducing the chronic shortage of kidneys available for transplantation.
When kidneys fail, dialysis can help. But a transplant is the more effective and better quality-of-life option, as long as the implanted organ functions well.
“I’m convinced that the general approach that some of these companies are taking is going to be effective.”
— MARTIN POLLAK
To assess the likelihood of this, clinicians rank potential transplant organs using a 10-factor scale. Because kidneys donated by African Americans have historically failed, on average, faster than those from other groups, one criterion is whether the donor is Black. “The way the calculation is done,” says Moxey-Mims, “this automatically downgrades the kidney.”
But small studies published since 2011 indicate that a major factor here is likely the APOL1 genotype. “Those Black donors who didn’t have two high-risk alleles — their donated kidney lasted as long as anybody else’s,” Moxey-Mims says.
These studies have already led some US transplant centers to refuse kidneys from people with high-risk APOL1 genotypes. But in a bid to unify transplant policy, Moxey-Mims is chairing an investigation charting the longevity of kidneys donated by African Americans in over 2,600 transplants according to the donors’ APOL1 genes.
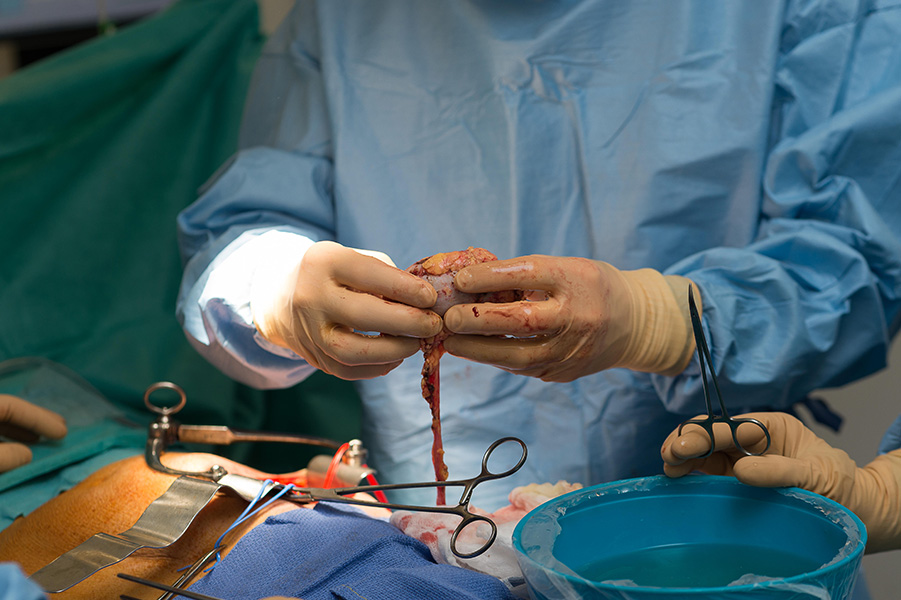
For people whose kidneys fail, the best option is a transplant. But in the United States, most people spend three to five years on the waiting list for a suitable donor organ.
CREDIT: BSIP SA / ALAMY STOCK PHOTO
Moxey-Mims hopes to publish preliminary results in 2025. If they confirm earlier results, APOL1 genotype might end up replacing race on the transplant checklist. “If it turns out that this person of African descent does not have two high-risk alleles,” she says, “then there’s no reason to discard that kidney or downgrade it.”
Since around 87 percent of Black Americans don’t have two copies of high-risk gene variants, this could significantly increase the number of available kidneys.
Ensuring a bright future
Inaxaplin is a “potentially amazing therapeutic,” says Neil Powe, a physician at the University of California, San Francisco, who researches kidney disease and racial health disparities. (Powe is not involved with the inaxaplin trials.) Clinical interventions that make specific use of APOL1, he says, open the door to furthering “precision equity,” in which disparities in diseases between groups are tackled by understanding the multiple causes — then identifying and addressing the factors that are most important to individuals.
But success will depend upon drugs reaching the right people — and reaching them before kidneys are damaged beyond recall. This, say researchers, will require effective genetic screens used in a socially sensitive manner, and people who are well but genetically at risk might benefit from regular urine protein measurements.
It is unclear how much the US racial disparity in kidney disease rates could be erased by APOL1 therapies. Friedman says he is most optimistic about the impact in young people, where kidney disease disparities are especially glaring.
And, of course, this is not just an American issue. Moxey-Mims recently attended a meeting in Ghana where local delegates wanted assurances that APOL1 drugs would be available across West Africa. She says they cited the pricing structures that pharmaceutical companies implemented to ensure HIV drugs are available in low- and middle-income countries as a model to ensure that APOL1 drugs reach as many people as possible.
Nor does the challenge end in West Africa. “It’ll need to be everywhere,” says Moxey-Mims, “everywhere the African diaspora is.”
10.1146/knowable-091824-1
TAKE A DEEPER DIVE | Explore Related Scholarly Articles