As a child, Cathryn Nagler broke out in hives when she ate eggs. She reacted to penicillin. Working in labs after college, she developed a severe allergy to mice that caused wheezing, swelling and trouble breathing — twice landing her in the emergency room.
Today, Nagler is an immunologist at the University of Chicago and is helping to pioneer an emerging research field: studying how bacteria in the gut can be harnessed to help people with food allergies.
It wasn’t personal experience with allergies that inspired her interest. Rather, it was an odd observation she made as a doctoral student in the 1980s. She was studying mice whose immune systems go haywire and attack the collagen protein inside their joints, causing severe arthritis. Scientists could jump-start the disease by administering a shot of collagen under the skin. But, curiously, when Nagler later fed the creatures collagen using a tube that snaked down into their stomachs, it had the opposite effect: The mice got better.
Decades on, this concept, called oral immunotherapy, has come into use as a treatment for food allergies, which affect an estimated 32 million people in the United States, including about two schoolchildren per classroom. Over the last ten years or so, some allergists have begun treating food allergy patients with small, regular doses of the offending food (or products made from it) to calm allergic responses. The approach stands to grow in popularity with the approval in January of a standardized version — a set of daily capsules to treat peanut allergy — by the US Food and Drug Administration.
But oral immunotherapy has downsides. The regimen can be nerve-racking, since it involves daily consumption of food that could kill. It doesn’t work for everyone and does little to fix the underlying disease. Success mostly means gaining the ability to safely eat several peanuts, for example, rather than reacting to a speck of peanut flour.
For some families, this modest gain is life-altering. Still, it is precarious: Patients must consume a bit of the food every day, or a few times a week, for the rest of their lives — or they could lose the protection.
So Nagler and several other researchers are working to find ways to treat food allergies more easily and durably. They’re targeting what they believe is a root cause — imbalances in the community of beneficial bacteria, or microbiome, that lives in our guts — in the hopes of resetting the immune system.

Cathryn Nagler discusses experiments with former research technician Elliot Culleen.
CREDIT: POLSKY CENTER / UNIVERSITY OF CHICAGO
Producing a microbiome-based treatment will be challenging, with many details to hash out, such as which microbes to provide and how best to deliver them. But the approach is gaining momentum. Last year, Nagler’s team and another group in Boston reported an important step forward: They prevented severe allergic responses in allergy-prone mice by supplying gut microbes from healthy, non-allergic human babies. “The data are sound, and they are very encouraging,” says pediatric allergist Jaclyn Bjelac of the Cleveland Clinic.
And in March, scientists reported finding large amounts of antibodies against peanut allergens in the stomach and gut of allergic patients, further supporting the idea that the gastrointestinal tract is a hotspot for food allergy regulation and treatment. Already, companies are testing several strategies.
It has long been a puzzle why one person tolerates a food while another is allergic but, as outlined in an article she coauthored in the Annual Review of Immunology, Nagler is convinced that the microbiome is key.
Birth of a hypothesis
Four years after finishing her graduate work, Nagler started running a lab at Harvard Medical School. She was studying inflammatory bowel disease, not food allergies, back then. But as research in the 1990s showed that inflammatory bowel disease was primarily caused by immune reactions against gut bacteria, she shifted her attention to the microbiome.
Then, in 2000, she came across an intriguing publication. It described a mouse model for peanut allergy that mimics key symptoms experienced by people. The mice scratch relentlessly. Their eyes and mouths get puffy. Some struggle to breathe — a life-threatening allergic response called anaphylaxis.
All of this happens after researchers feed the mice peanut powder. “That caught my eye,” Nagler says. It ran counter to her earlier findings with the arthritic mice, where feeding collagen calmed the immune reaction. Why the difference?
The peanut-allergy mice, another report showed, had a genetic glitch that damages a receptor called TLR4 that sits in the membranes of immune cells and recognizes microbes. It looked as though the peanut-allergy mice lacked the normal cross talk that takes place between gut microbes and immune cells.
“That was my lightbulb moment,” Nagler says. Perhaps the trillions of microbes that live in us suppress immune responses to food by stimulating the TLR4 receptor. And perhaps perturbations in that teeming microbiome alter the suppression and cause a rise in allergies.
The idea meshes with historical trends. As societies modernized, people moved to urban areas, had more babies by cesarean section, took more antibiotics and ate more processed, low-fiber foods — all of which shake up microbiomes. The timing of these lifestyle shifts parallels the observed increase in food and other types of allergies, whose steep rise over a generation points to some environmental cause.
Rates of food allergies have risen steeply over the last few decades, in line with a variety of changes in the way we live. A lot of these aspects of a modern lifestyle — such as fatty, low-fiber diets and the use of antibiotics — have potential to affect the types of microbes that live in our guts. Some scientists think that such microbiome shifts are making allergies more likely.
In 2004, Nagler and her coworkers published a report showing that peanuts provoked anaphylaxis only in mice with a mutated TLR4 receptor, not in genetically related strains with a normal TLR4. The difference disappeared when the scientists wiped out populations of gut bacteria with antibiotics. Then, even normal mice became susceptible to food allergies, implying that bacteria are at the heart of the protection.
Nagler’s lab has been working ever since to identify which bacteria are helpful, and to understand how they regulate allergic responses.
Early effects
In their work, Nagler’s team focused on Clostridia and Bacteroides — two major groups of bacteria in the human gut. Working with mice bred in a germ-free environment and thus without any microbiome at all, the team found that Clostridia, but not Bacteroides, prevented food-allergic responses when introduced into the guts of the squeaky-clean mice.
There’s a potential explanation: Mice colonized with Clostridia bacteria had more regulatory T cells, a type of cell that dampens immune responses. The Clostridia mice also produced more of a molecule called IL-22 that strengthens the intestinal lining. A new theory began to emerge: If protective microbes are missing, the gut barrier weakens, allowing food proteins to seep into the bloodstream and potentially trigger allergic responses.
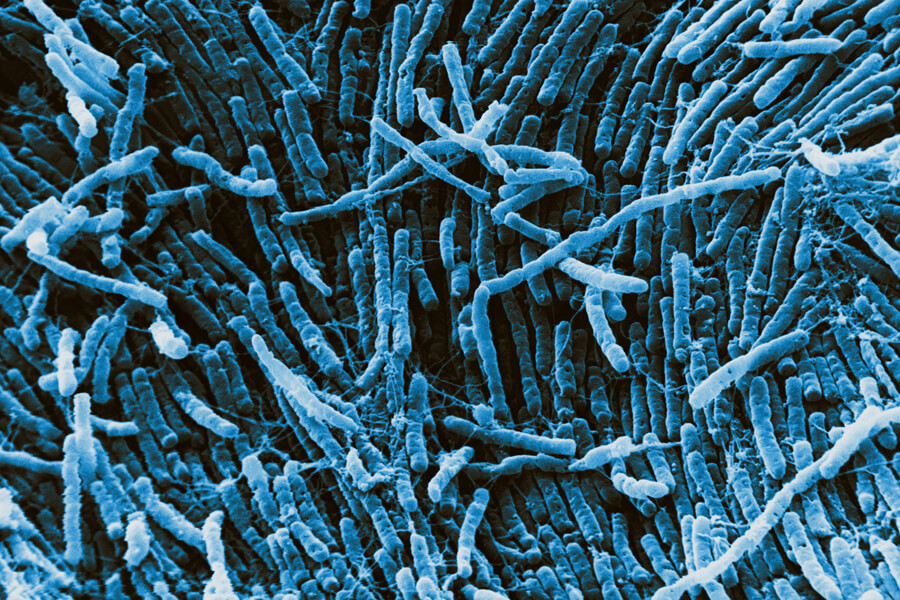
This color-enhanced scanning electron micrograph shows bacteria of a type called Clostridia, which live in mammalian guts. Certain Clostridiabacteria have been linked to protection against food allergies.
CREDIT: DAVID M. PHILLIPS / SCIENCE SOURCE
This reasoning jibes well with the curious observation that top food allergens (certain proteins found in milk, eggs, peanuts, tree nuts, soy, wheat, fish and shellfish) bear little biochemical resemblance to each other. What they do have in common is the ability to remain intact in the digestive tract, which normally breaks food into small pieces that the body absorbs as nutrients. “That seems to be what makes peanut the champion — its ability to resist degradation in the gut,” Nagler says.
Studies have further solidified the link between gut bacteria and food allergies and suggest that the microbiome’s impact comes early in life. Analyzing feces of healthy babies and those with egg or milk allergies, researchers showed that allergic and nonallergic infants had different communities of gut bacteria.
Another study tracked 226 children with milk allergy from infancy to age 8. The scientists found that certain bacteria, including Clostridia, were enriched in stool samples from 3- to 6-month-old infants who eventually outgrew their allergy, compared to those who remained allergic. The scientists didn’t see the same difference between these groups in older babies, suggesting that allergy-protective microbes may only act early in life.
“All of this points to the concept of a window of opportunity in terms of prevention,” says study leader Supinda Bunyavanich, a pediatric allergist at the Icahn School of Medicine at Mount Sinai in New York City.
Causal evidence
From birth, our immune systems get schooled in life-or-death choices. They learn to kill germs, tumors and dying cells. Much else in their surroundings they must learn to leave alone — nerve fibers, bone tissue, proteins from milk and cookies consumed at snack time. Mouse studies published in 2019 by Nagler’s lab and another team argue convincingly that gut microbes cultivate this critical immune decision-making.
In one of the studies, Nagler and coworkers collected gut bacteria from the feces of healthy and milk-allergic babies and put those collections of microbes into the digestive tracts of germ-free mice. They found that gut bacteria from healthy babies protected mice against allergic responses to milk, whereas microbes from allergic infants didn’t.
Using mathematical and computer science techniques to analyze the results, the team identified bacterial strains that were present in healthy but not allergic babies. They also examined gene activity in cells lining the intestines — certain gene patterns are characteristic of a healthy gut barrier — and looked for microbes whose presence correlated with a healthy barrier.
To understand how gut bacteria influence food allergies, researchers collected feces from four healthy babies and four babies allergic to cow's milk and transferred these microbe-rich samples into germ-free mice. Later, they exposed the mice to cow’s milk allergen and measured allergic responses in the animals. The team then analyzed gene activity in cells lining the animals’ small intestines — which interact heavily with gut microbes. They saw notable differences between the differently treated mice. In the above chart, color and intensity of the boxes indicate higher (red) or lower (blue) activity of genes. The genes’ names are listed to the right of the grid. Some genes were more heavily active in mice that got microbes from healthy babies (“Up in healthy”), while other genes showed more activity in mice that got microbes from allergic babies (“Up in CMA”) — or vice versa (bottom two sets of rows). By mapping and comparing these effects, the researchers deduced which microbes seemed associated with protection against milk allergy (not shown).
One Clostridia species, Anaerostipes caccae, popped out of both analyses. When the scientists transferred A. caccae alone into germ-free mice, it seemed to mimic the protection imparted by a full, healthy microbiome.
The other team, led by Rima Rachid and Talal Chatila at Boston Children’s Hospital, took a similar approach using hyper-allergic mice, finding that the single species Subdoligranulum variabile and a set of Clostridia species prevented allergic responses. Regulatory T cells were key to the response and were spurred into action by the microbes.
These and other studies clearly show that the microbiome is important for preventing food allergies and inducing tolerance, says Carina Venter, a research dietician at the University of Colorado in Denver who is studying links between maternal diet during pregnancy, microbiomes of infants and risk for eczema and allergies. But, she says, “how that microbiome should look in terms of diversity and in terms of specific strains, we just don’t know.”
Trials and questions
The many unknowns leave a quandary for researchers hoping to develop better treatments for food allergies: Is it better to supply a full, healthy microbiome, or to replenish just a few helpful microbes? “I scratch my head every day thinking about this,” Rachid says.

Certain foods are far more likely than others to cause allergies: eggs, shellfish, nuts, peanuts, soy, fish, milk and wheat. These foods, though very different, all contain proteins that are resistant to digestion.
CREDIT: SCIENCE PHOTO LIBRARY / SCIENCE SOURCE
She’s leading a clinical study to test the first possibility. In this small trial, adults with peanut allergies will swallow pills containing a full slate of gut bacteria from healthy donors pre-screened for safety by the nonprofit stool bank OpenBiome. The approach, known as fecal transplantation, is not FDA-approved but is increasingly used to treat severe intestinal disorders with the aim of fixing diseased microbiomes by infusing healthy, balanced ones.
Other trials are also underway. Using the protective strains identified by the Boston team, Pareto Bio of La Jolla, California, is developing a live microbial product to treat food allergies. Another company, Vedanta Biosciences of Cambridge, Massachusetts, is developing a probiotic capsule that contains a mix of Clostridia strains selected for their ability to induce regulatory T cells. Vedanta is testing the capsules as an add-on to oral immunotherapy in adults with peanut allergies.
A third company, Prota Therapeutics of Melbourne, Australia, is commercializing a similar strategy combining peanut oral immunotherapy with a probiotic — in their case, a Lactobacillus strain commonly prescribed for gastrointestinal problems.
Administering whole microbiomes from donors is not without risk: Four patients have been hospitalized, and one died, from serious infections linked to stool transplants. So some researchers think it may be better to use precisely defined species. Though this risks weakening the benefit, “you’re less likely to induce unanticipated problems,” says Wayne Shreffler, who directs the food allergy center at Massachusetts General Hospital in Boston and is leading the Vedanta study.
But there’s one challenge shared by all microbiome-modulating approaches: getting new microbes established when someone already has a microbiome in place, even an unhealthy one. Traditionally, patients receive antibiotics to help new bacteria gain a foothold. But maybe there’s another way. A start-up that Nagler cofounded with University of Chicago biomolecular engineer Jeff Hubbell — ClostraBio — is developing a therapy that combines live bacteria with a key microbial metabolite, butyrate.
The chemical is known to enhance gut barrier function and may also have antimicrobial effects, which could help create a niche for the added microbes. ClostraBio plans to launch its first human trial by 2021, Nagler says.
Over the next few years, researchers will learn more about harnessing the microbiome to fight food allergies. It won’t be easy. Genetics, diet, environmental exposures: All influence allergy risk. “It’s a big puzzle,” says Bunyavanich. The microbiome is only one piece of it — but she, Nagler and others are betting it will turn out to be a big one.




