How stress shapes cancer’s course
Studies show psychological strain can accelerate tumors — could beta blockers slow them down?
Support sound science and smart stories
Help us make scientific knowledge accessible to all
Donate today
About two millennia ago, the Greek physicians Hippocrates and Galen suggested that melancholia — depression brought on by an excess of “black bile” in the body — contributed to cancer. Since then, scores of researchers have investigated the association between cancer and the mind, with some going as far as to suggest that some people have a cancer-prone or “Type C” personality.
Most researchers now reject the idea of a cancer-prone personality. But they still haven’t settled what influence stress and other psychological factors can have on the onset and progression of cancer. More than a hundred epidemiological studies — some involving tens of thousands of people — have linked depression, low socioeconomic status and other sources of psychological stress to an increase in cancer risk, and to a worse prognosis for people who already have the disease. However, this literature is full of contradictions, especially in the first case.
In recent decades, scientists have approached the problem from another angle: experiments in cells and animals. These have revealed important mechanisms by which stress can alter tumors, says Julienne Bower, a health psychologist at UCLA who coauthored a 2023 article on the connection between the brain and the immune system in diseases, including cancer, in the Annual Review of Clinical Psychology. Such studies are showing that “psychological factors can influence aspects of actual tumor biology,” she says. On the flip side, studies in people and animals suggest that blocking the chemical signals of stress may improve cancer outcomes.
Today, a growing number of researchers think that psychological factors can influence cancer’s progression once someone has the disease. “I don’t think anyone appreciated the magnitude by which even mild stress, if it’s chronic, can have such a negative influence on cancer growth,” says Elizabeth Repasky, a cancer immunologist at the Roswell Park Comprehensive Cancer Center in Buffalo, N.Y.
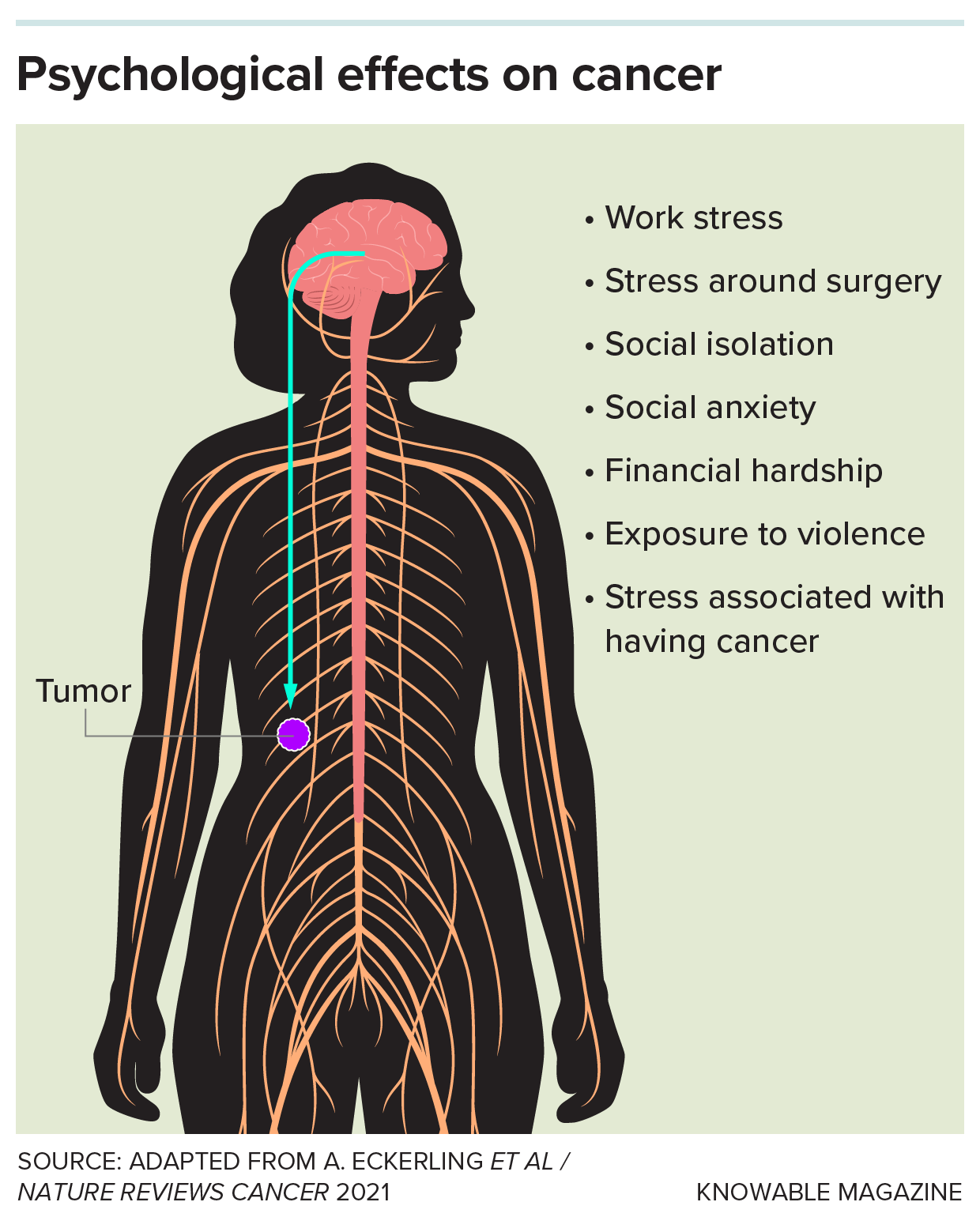
People with cancer may experience a wide range of stressors, both related and unrelated to the disease. A growing body of evidence suggests that these may influence how a tumor grows and spreads.
A viral beginning
New interest in the relationship between stress and cancer growth emerged in part from research into how stress affects the body’s response to human immunodeficiency virus (HIV). In the 1990s and early 2000s, genomics researcher Steve Cole and his team at UCLA investigated why people infected with HIV who were under high stress tended to have worse outcomes, including larger viral loads and poorer responses to antiretroviral drugs.
Cole’s team discovered several routes through which stress could worsen HIV infections. In monkeys, they found, the lymph nodes of stressed animals had many more connections to sympathetic nerve cell fibers — which execute the body’s fight-or-flight response — than the nodes of unstressed monkeys. Lymph nodes contain immune cells, and the nerve fibers reduced the antiviral function of these cells, which, in turn, led to an increase in the replication of a version of HIV that infects monkeys and apes.
Lymph nodes, in addition to housing immune cells, also act as the body’s drainage system, flushing away toxins through a network of tissues, organs and nodes called the lymphatic system. Importantly, cancer cells can hijack this system, using it to travel through the body. Erica Sloan, a postdoctoral trainee of Cole who was involved in the HIV work, wondered whether stress, via the sympathetic nervous system, might also affect lymph nodes in those with cancer.
Sloan, now a cancer researcher at Monash University in Australia, went on to discover in mice that chronic stress increases the number of connections between the lymphatic system and breast tumors, making the cancer cells more likely to spread. Strikingly, treatment with a drug — a beta blocker that blunts the activity of key molecules of the sympathetic nervous system such as norepinephrine — prevented these effects.
Research by other groups has shown that stress can lead to molecular changes, particularly within the immune system, that influence how cancer progresses. Some of this work suggests that, when stress leads to inflammation — a broad immune reaction typically brought on by injuries and infections — it can boost the growth of tumors.
Stress can also impair the activity of immune cells that play an active role in fighting cancer. In the early 2000s, research by University of Iowa behavioral scientist Susan Lutgendorf and her colleagues found that in patients with ovarian cancer, depression and anxiety were associated with impaired tumor-fighting immune cells. In another study of people with ovarian cancer, the researchers found that poor social support was linked to higher levels of a growth factor that stimulates blood vessel growth around tumors. This growth, called angiogenesis, enables new blood vessels to supply nutrients to tumors and — like the lymphatic system — provide pathways through which cancer cells can spread to other parts of the body.
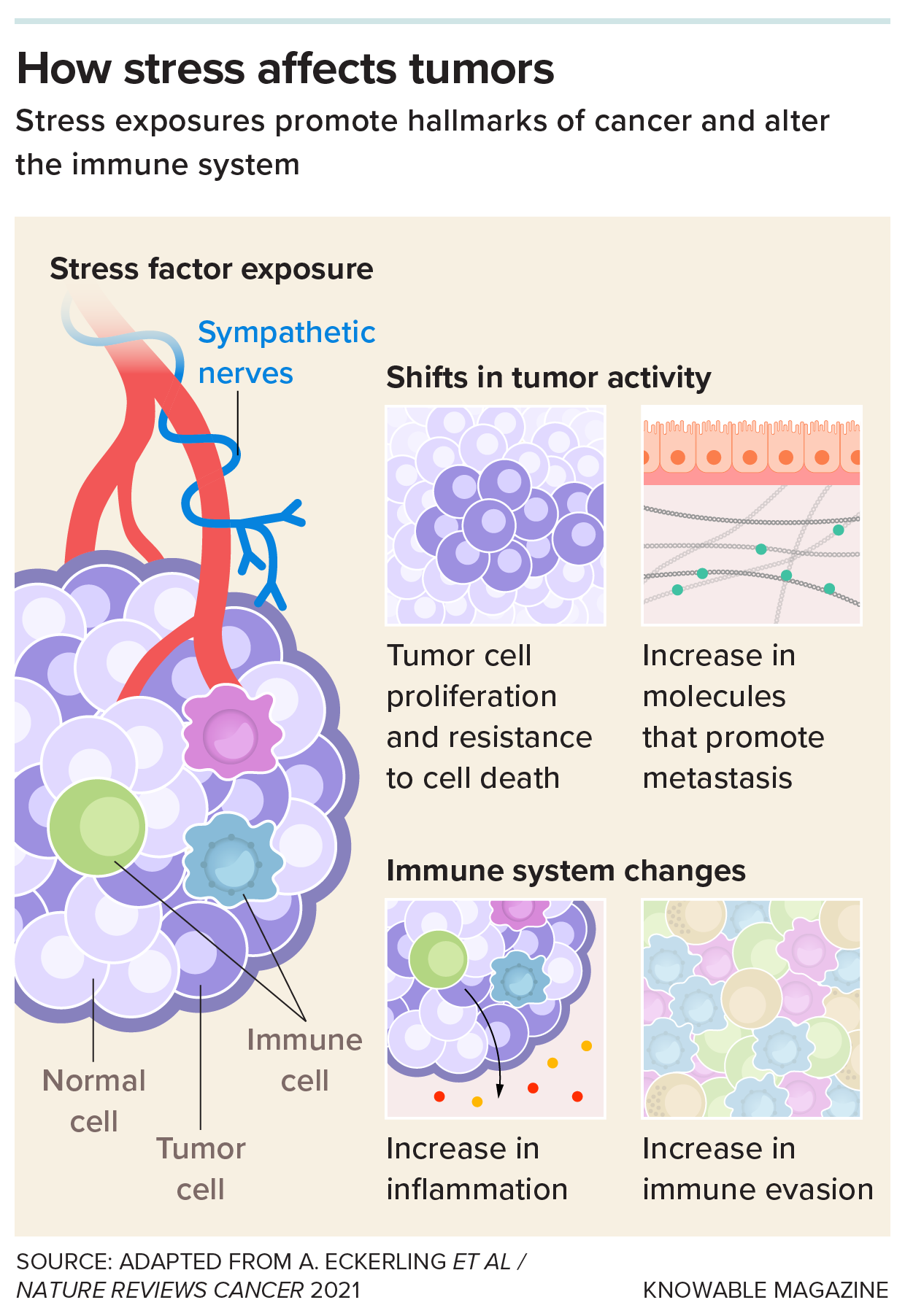
The sympathetic nervous system carries signals from the brain to sites around the body, including tumors. These signals can influence tumor cells in a variety of ways that promote cancer, studies show. Blocking the activity of key molecules in this system with beta blockers may help counteract some of these effects.
Lutgendorf and her colleagues have since found that stressful situations have a similar effect on mice with ovarian cancer, enhancing tumor angiogenesis and cancer spread. Equally important, they’ve found that these effects can be reversed with beta blockers. Other groups have found similar effects of blocking stress signals on other types of cancer in rodents, including blood and prostate cancer. In addition, researchers have found that increasing levels of stress hormones such as norepinephrine and cortisol in mice can make previously dormant cancer cells more likely to divide and form new tumors.
Studies like these are revealing that stress can trigger a cascade of biochemical changes and alter a cancer cell’s environment in a way that may promote its spread. “Stress signaling and stress biology really have an impact on most — if not all — of these processes,” says Jennifer Knight, a cancer psychiatrist at the Medical College of Wisconsin.
Blocking stress signals
If stress can make cancer worse, how can the process be stopped? Little by little, new treatments are emerging.
For about half a century, clinicians have used beta blockers to treat hypertension. By scouring data from patient registries, researchers found that people with cancer who already had been taking certain kinds of beta blockers at the time of diagnosis often had better outcomes, including longer survival times, than those who were not on the medicines.
Over the last few years, several clinical trials — most of which are small and early-stage — have directly tested whether beta blockers could benefit people with cancer. In one pair of studies, a research team led by neuroscientist Shamgar Ben-Eliyahu at Tel Aviv University, administered the beta blocker propranolol along with an anti-inflammatory drug to people with colorectal or breast cancer five days before surgery. The team chose this timing because earlier research had shown that while surgery is an opportunity to remove the tumor, it can also paradoxically provide the chance for the cancer to spread. So blocking any potential effects of stress on cancer spread, they reasoned, could be crucial to a patient’s long-term prognosis.
These trials, which involved dozens of patients, revealed that the tumor cells of those who received the drugs showed fewer molecular signs of being able to spread — a process known as metastasis — less inflammation, and an increase in some tumor-fighting immune cells. For colorectal cancer patients, there were also hints that the intervention could reduce cancer recurrence: Three years after the procedure, cancer returned in two of the 16 patients who received the drugs, compared to six of 18 patients who didn’t receive those meds.
Other studies have assessed the effect of using beta blockers alone, without anti-inflammatory drugs. In 2020, Sloan and her colleagues published a study including 60 breast cancer patients, half of whom were randomly assigned to receive propranolol a week before surgery, while the other half received a placebo. They, too, found that tumor cells from patients who received beta blockers had fewer biomarkers of metastasis.
Stress-reducing beta blockers may also benefit other cancer treatments. In a 2020 study, Knight and her team looked at the effect of beta blockers in 25 patients with multiple myeloma who were receiving blood stem cell transplants. Patients who took beta blockers had fewer infections and faster blood cell recovery — although the study was too small to properly evaluate clinical outcomes. And in a small study of nine people with metastatic skin cancer, Repasky and her colleagues found hints that beta blockers might boost the effectiveness of cancer immunotherapy treatments.
Breast cancer cells grown in a dish. In small clinical trials, researchers have found that tumors in breast cancer patients treated with beta-blockers have fewer biomarkers of metastasis, less inflammation and an increase in tumor-fighting immune cells.
CREDIT: TOM MISTELI, KAREN MEABURN / NCI CENTER FOR CANCER RESEARCH
While studies on beta blockers are promising, it’s not clear that these drugs will improve outcomes in all kinds of cancers, such as lung cancer and certain subtypes of breast cancer. Some patients can react badly to taking the medications — particularly those with asthma or heart conditions such as bradycardia, in which the heart beats unusually slowly.
And, crucially, the drugs only block the endpoint of stress, not its cause, Repasky says. They will therefore likely need to be combined with mindfulness, counseling and other stress-reducing strategies that get closer to the root of the problem.
Such interventions are also in the works. Bower and her team have conducted clinical trials of mind-body interventions such as yoga and mindfulness meditation with breast cancer survivors, to improve health and promote lasting remission. They’ve found that these therapies can decrease inflammatory activity in circulating immune cells, and they speculate that this may help to reduce tumor recurrence.
Breaking barriers
Ultimately, bigger clinical trials are needed to firmly establish the benefits of beta blockers and other stress-reducing interventions on cancer survival outcomes — and determine how long such effects might last. The timing of treatment and the type of cancer being treated may play a role in how well such therapies work, researchers say. But lack of funding has been a barrier to conducting the larger follow-up studies needed to answer such questions. The work isn’t yet backed by pharmaceutical companies or other organizations that support large studies in oncology, Knight says.
And for now, whether stress can increase a person’s risk of developing cancer in the first place, as the ancient Greeks once postulated, remains a mystery. Population studies linking stress to cancer risk are often complicated by other factors, such as smoking, poor nutrition and limited access to health care.
“We have no definitive way of saying, ‘If you’re stressed out, you’re going to develop cancer,’” says Patricia Moreno, a clinical psychologist at the University of Miami Miller School of Medicine and coauthor of an article in the 2023 Annual Review of Psychology about stress management interventions in cancer.
But for people who already have a cancer diagnosis, many researchers argue that the evidence is strong enough to include stress management in clinical practice. On average, cancer patients do not receive psychological therapies that can reduce stress at the level for which they are needed, says Barbara Andersen, a clinical psychologist at Ohio State University. Although they won’t be necessary for every patient, many can benefit from mind-body interventions, she says. “I’m not saying they should be a first priority, but they shouldn’t be the last.”
10.1146/knowable-040725-2
TAKE A DEEPER DIVE | Explore Related Scholarly Articles