How cancer cells travel to new tissues and take hold
Understanding these astonishing migrations through the human body, known as metastases, could suggest novel treatments
Support sound science and smart stories
Help us make scientific knowledge accessible to all
Donate today
Back in 2014, a woman with advanced cancer pushed Adrienne Boire’s scientific life in a whole new direction. The cancer, which had begun in the breast, had found its way into the patient’s spinal fluid, rendering the middle-aged mother of two unable to walk. “When did this happen?” she asked from her hospital bed. “Why are the cells growing there?”
Why, indeed. Why would cancer cells migrate to the spinal fluid, far from where they’d been birthed, and how did they manage to thrive in a liquid so strikingly poor in nutrients?
Boire, a physician-scientist at Memorial Sloan Kettering Cancer Center in New York, decided that those questions deserved answers.
The answers are urgent, because the same thing that happened to Boire’s patient is happening to increasing numbers of cancer patients. As the ability to treat initial, or primary, tumors has improved, people survive early rounds with cancer only to come back years or decades later when the cancer has somehow resettled in a new tissue, such as brain, lung or bone. This is metastatic cancer, and it’s the big killer — while precise numbers are scarce, anywhere from half to the large majority of cancer deaths have been attributed to metastasis. Offering people more options and hope will mean understanding how those cancers successfully migrate and recolonize.
The prevalence of metastasis belies the arduous journey that cancer cells must make to achieve it. A cell that arises in, say, the breast, is well-adapted to live there: to eat the fatty acids available to it, to resist local threats, and to grow there in a solid tumor. If it manages to escape into the bloodstream, it finds itself zipping along at up to 40 centimeters per second with shear stresses sufficient to rip it apart. Should it survive that odyssey and land in a new tissue — say, the brain or spinal fluid — the environment is totally different yet again. The foods the cell is accustomed to may be absent; immune cells or other novel environmental molecules may attack. For a cell to manage this trip, and then adapt to a new environment, is truly a Herculean task.
“It is not easy and trivial,” says Ana Gomes, a cancer biologist at the Moffitt Cancer Center in Tampa, Florida. “It’s just against everything in the nature of these cells.”
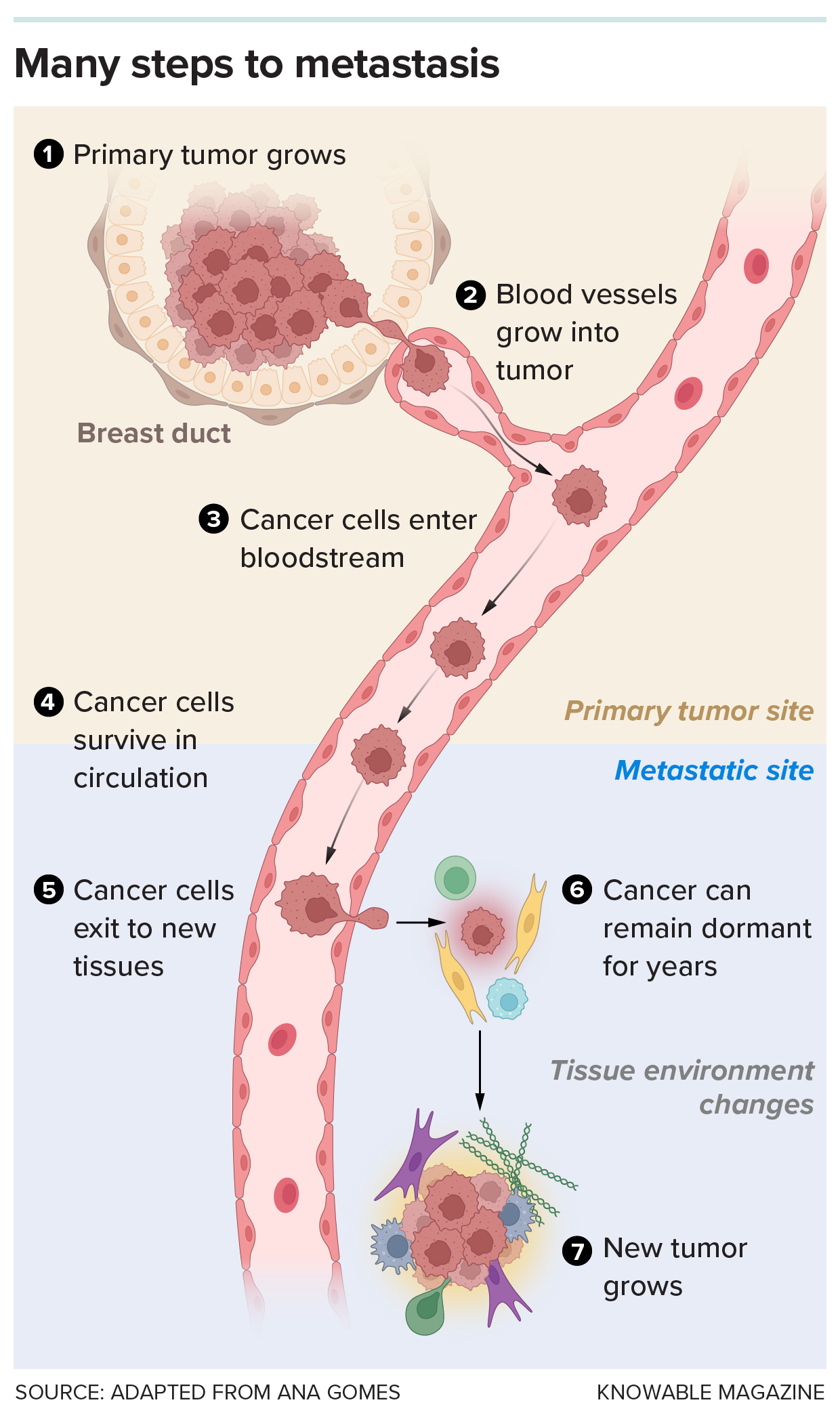
Moving to a new site and forming a new tumor is an arduous journey that few cells can complete. A cell must exit the initial tumor, survive the bloodstream and enter a new tissue. Even then, the cell may remain dormant for a time, until the environment can support its division and growth to create a new tumor.
No wonder that, even though tumors regularly shed cells, most escapees perish or languish without successfully establishing themselves as metastases. “Personally, I think metastasis is an accident,” says Matthew Vander Heiden, a physician-scientist and director of the Koch Institute for Integrative Cancer Research at MIT. “It’s really, really inefficient.”
The few cells that manage this epic feat are resilient and flexible in how they feed themselves and process the molecules around them. They may tweak their biochemistry to evade local dangers, or to get the fuel they need in sparse environments. Some even send signals ahead to modify the organ where they’ll land, creating a cushy nest with a food supply ready for when they arrive. “Metabolic changes help these cells to face all this challenge,” says Patricia Altea-Manzano, a biomedical researcher at the Andalusian Molecular Biology and Regenerative Medicine Center in Seville, Spain.
Such findings suggest ways that metastasizing cells, because they’re so different from the original tumor, might be vulnerable to new kinds of treatment. Someday doctors might not have to wait for metastasis to take hold before they block or slow cancer’s spread: “That is a very big opportunity,” Gomes says.
Novel adaptations
Metabolically, there’s no place like home: Cancers tend to do best in the tissues where they initially grow, Vander Heiden’s group has found. And when they do move, these primary tumors have preferred target sites — prostate cancers tend to move into bone, for example.
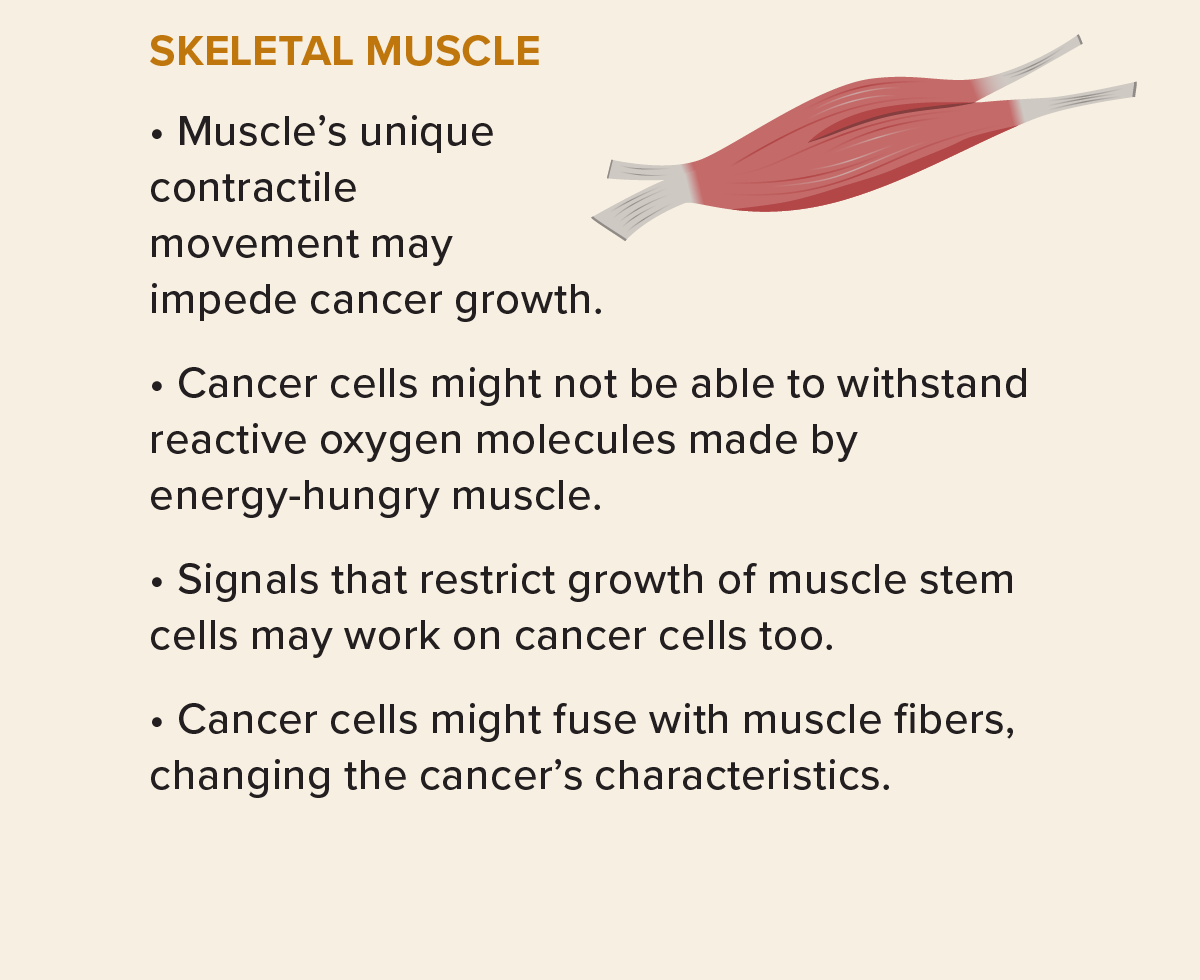
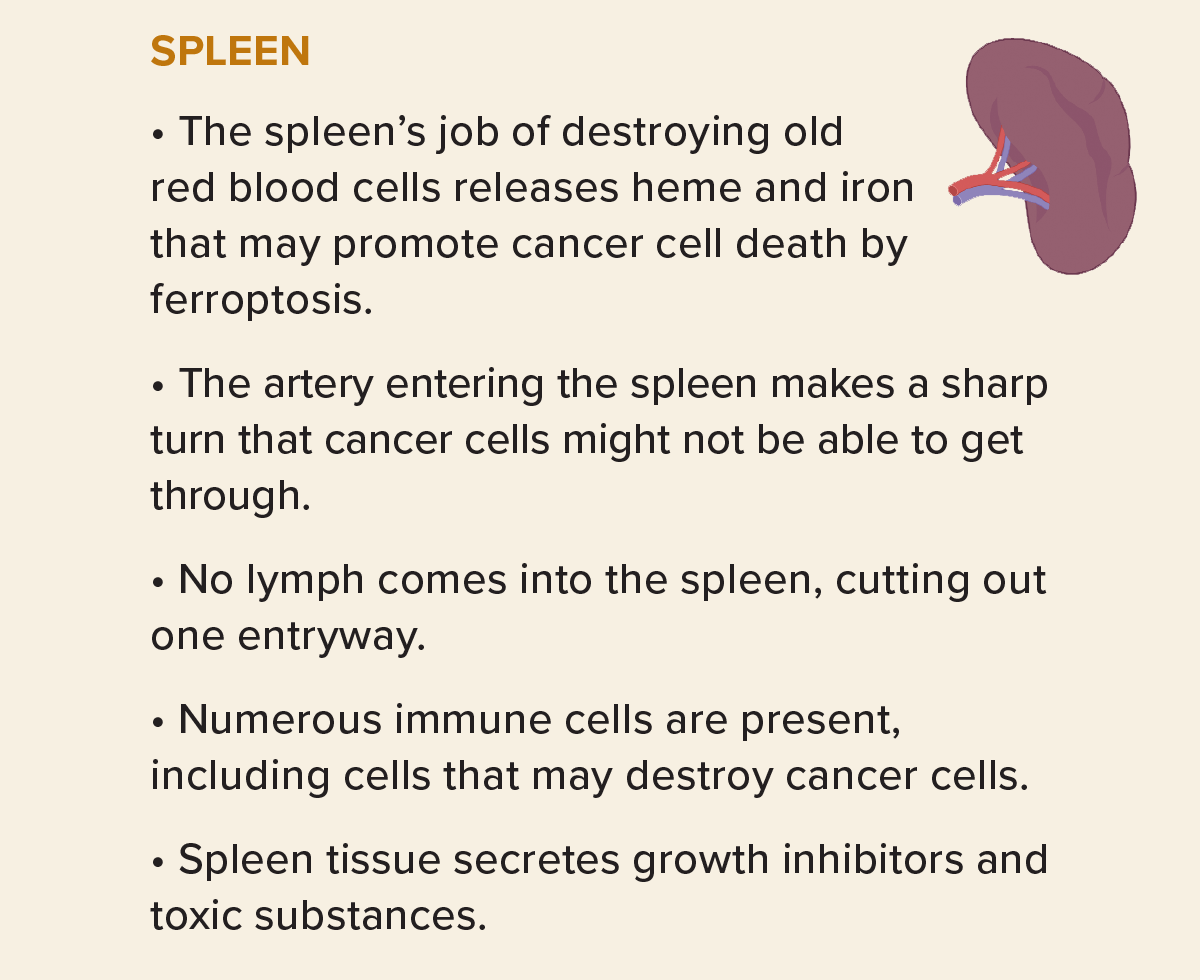
Some cells, however, will land in a place to which they are very unlikely to ever adapt: Certain sites, such as the spleen and skeletal muscles, seem to resist metastasis, and there are many possible reasons. Muscle cells, for example, use tons of energy, causing their mitochondria to release lots of a side product of energy processing: reactive oxygen species such as hydrogen peroxide. These vigorously oxidizing molecules are toxic, but local muscle cells can handle them. Yet even though plenty of tumor cells reach the skeletal muscle via the blood that copiously feeds it, they rarely take hold there, stymied, researchers suspect, by those reactive oxygen molecules.
But adaptation to other novel environments is possible, as Vander Heiden discovered when his group implanted human breast cancer cells into either mammary fat or the brains of mice. Though the brain lacks the kinds of fat building blocks — fatty acids — that breast cancer cells are accustomed to eating, when the cells were dropped into the brain, they adjusted to manufacture their own fatty acids.
The scientists then treated the mice with a drug that blocks fatty acid synthesis, the cancer cells in brain tissue grew at half-speed (the breast cells in the mammary fat continued to grow unbothered). Vander Heiden has consulted for companies that are in the early stages of exploring this approach as a treatment.
Sometimes, tumors can even prime a foreign site for their arrival, in a process some researchers call “education of the metastatic niche.” Cancers shed not only cells, but also hormones, DNA and little fatty bubbles called vesicles into the blood and lymph. These bubbles can contain chemical messages, and when these or other signals reach far-off organs, they can reshape the tissues to the tumor cells’ specifications. That “education” helps set up metastasizing cells to thrive in a new location says Gomes.
Even microbes can get in on the act: In the case of colorectal cancer, bacteria from the intestines teach the liver to receive metastatic cancer cells. The gut bacteria colonize the intestinal tumor, then break through the multilayer barrier that normally keeps gut contents away from the rest of the body. Then the bacteria can go into the liver, where they induce inflammation in the organ. This creates a pro-tumor environment, so cancer cells that arrive later are able to settle in.
The fatty acid connection
Altea-Manzano studied this priming process during her time as a postdoctoral scholar with cancer biologist Sarah-Maria Fendt at the VIB-KU Leuven Center for Cancer Biology in Belgium. In this case, it was the lungs that were being primed by tumors residing elsewhere. And much as Vander Heiden observed with breast cancer metastasis to the brain, access to fatty acids was a key factor — specifically, the fatty acid palmitate, whose functions include serving as an energy source and as a component of cellular membranes.
The lungs are already awash in a fat-rich material called surfactant, which coats the lungs’ interior and keeps the tissue from collapsing. When the researchers fed mice a high-fat diet, the levels of palmitate and other fatty acids in the lungs rose. And when the researchers injected mouse mammary (breast) cancer cells into the blood of those mice, the high-fat diet resulted in more than twice as much metastasis to the lung.
To check whether tumor cells were secreting something that primed the lungs to host them, Altea-Manzano and colleagues grew pieces of mouse mammary tumor in a dish, then collected the liquid containing all the cellular secretions. When they injected this cell-free soup into mice, it boosted the palmitate levels in the lungs; if they also injected cancer cells, this treatment increased the level of lung metastases by those cells, too. Some ingredient made by the cancer cells cultured in that lab dish was sending the lungs a message: Make more palmitate. (The scientists still aren’t sure what the signaling substance is.)
The result is that if a breast cancer cell lands in the lungs, it finds a fatty, ready-made feast to nosh on. To make the most of the new menu, however, a newly arrived breast cancer cell will have to alter its cell chemistry. It does that by changing its mitochondria so they can take up more palmitate. In experiments with mice, blocking that change interfered with metastasis, no matter how much palmitate was present. It might do the same in human patients, speculates Altea-Manzano, who with Fendt and others coauthored a discussion of metabolic changes that might promote or thwart metastasis for the 2024 Annual Review of Cancer Biology.
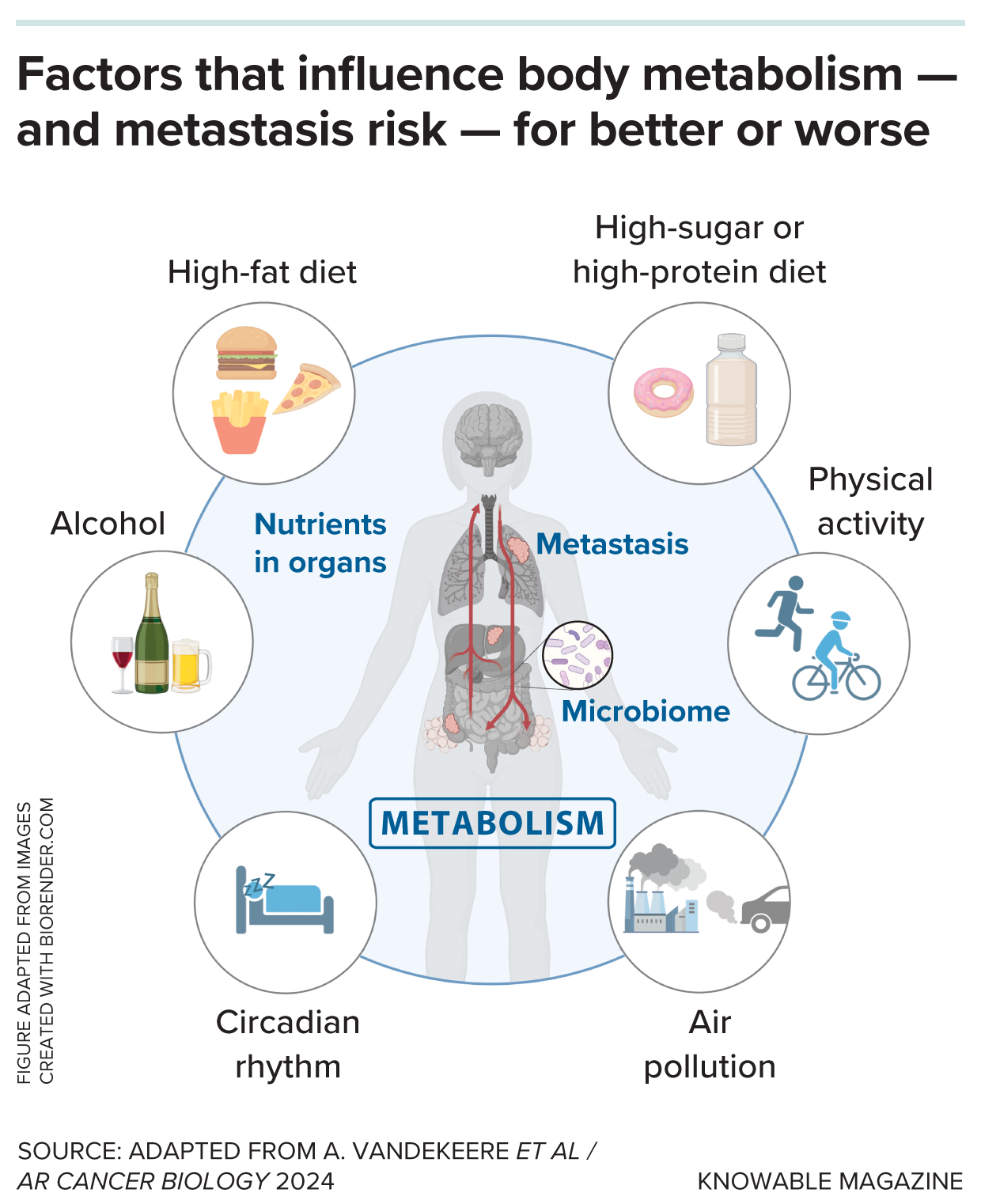
A person’s lifestyle as well as their environment can influence their metabolism and microbiome. That, in turn, can be a factor in the success or failure of cancer to metastasize. But the relationships are complex: Things that seem good for metabolism on the surface — such as antioxidants — aren’t always things that directly counter cancer spread.
Knowing the enemy
In addition to fat-rich places like the lungs, cancers can adapt to surprisingly challenging locales, such as the barren wasteland that is the spinal fluid surrounding the brain and spinal cord.
Most places in the body where tumors originate are replete with nutrients: fats, amino acids, oxygen, metals — all the foodstuffs a rapidly growing cell needs. In contrast, “the brain is kind of a metabolic princess,” says Boire. “It prefers glucose only, please.”
Not only is there precious little to eat, but cancer cells will find themselves surrounded by support cells of the nervous system and resident immune cells, both of which spew out anti-tumor agents.
Boire’s work focuses on the spinal fluid. It’s a clear liquid devoid of many nutrients, and yet metastasis to the spinal fluid happens in some 5 percent to 10 percent of solid-tumor patients, and it usually kills within months. For Boire, this makes such a cancer “a worthy adversary … It’s totally evil.”
To learn how such an evil cell survives, Boire and colleagues examined metastatic cells from five patients in whom breast or lung cancers had taken over the spinal fluid. These cells had all ramped up a biochemical system that sops up iron, a necessary metal to produce energy and more cell parts. As one part of the system, the cells secreted a protein called lipocalin-2 that collects the sparse iron in the environment; for the other part, they made a protein called a lipocalin-2-iron transporter that pulls the iron-lipocalin-2 complex into the cells.
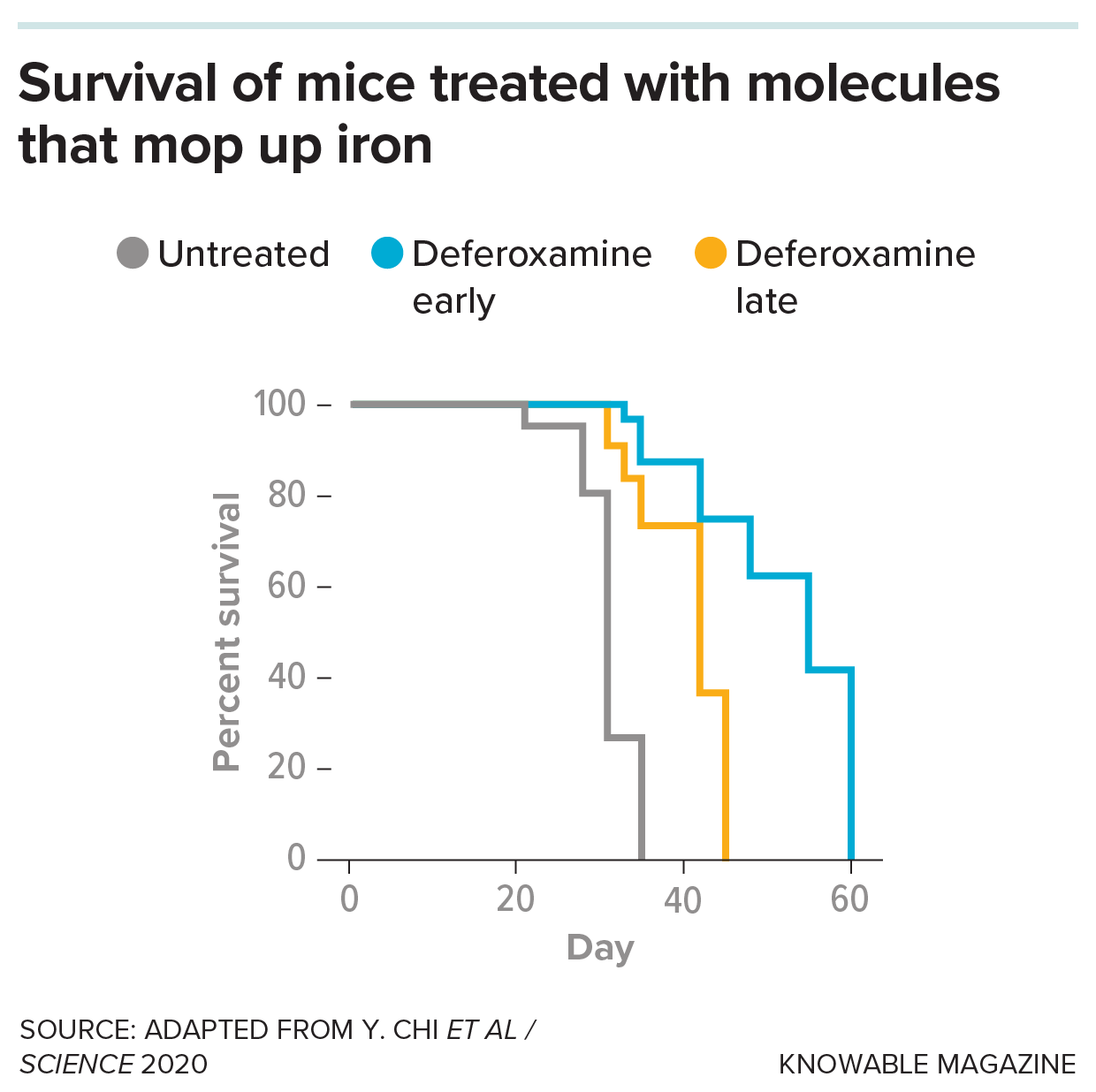
Mice studied as models for metastasis to the spinal fluid normally all die within fewer than 40 days. But when scientists treated the mice with a drug, deferoxamine, that prevents the cancer from accessing iron, they live for longer.
Studying the process further in mice, Boire’s team discovered that the cancer cells boost their iron collection in response to inflammatory molecules produced by local immune cells. The cancer cells then slurp up so much iron that the immune cells can’t meet their own needs for the metal. “They’re like the original jerks at the buffet,” says Boire. “You know these guys — they’re taking everything you want for themselves.”
To starve out these cellular creeps, the researchers treated mice with a molecule called deferoxamine that snatches the iron before lipocalin-2 has a chance to grab it. Sure enough, the iron levels in the cancer cells dropped. Moreover, the mice survived nearly twice as long as animals who didn’t get the treatment.
Boire has begun testing deferoxamine in a few dozen patients who have metastatic cancer in the spinal fluid and expects to publish results soon.
She notes that the treatment doesn’t act directly on the cancer but changes its environment so it can’t fulfill its needs. “It kind of opens up this idea — there are other ways of targeting cancer cell growth,” she says.
Stress points
In addition to food, traveling cancer cells need protection from changes to their metabolism in new environments. Metastasis itself seems to cause cancer cells to generate reactive oxygen species, which can kill them from within, says Sean Morrison, a cancer biologist at the University of Texas Southwestern Medical Center in Dallas.
His team studies this metastasis roadblock by injecting human melanoma cells into mice. The scientists can put the cells right under the skin where they should be comfortable, or stick them into other places, such as the bloodstream or spleen, to see if they can achieve metastasis.
In the skin, melanoma cells don’t experience much oxidative stress. But melanoma cells in the blood or other organs experience stress from higher levels of reactive oxygen molecule levels. It could be that higher levels of iron and oxygen in places like the blood drive biochemical changes that produce these dangerous molecules, Morrison suggests.
Oxidative stress kills wandering melanoma cells by a process called ferroptosis, in which polyunsaturated fatty acids in the cancer cell membrane react with iron. “It’s like a grease fire starting in the cancer cells as they’re trying to migrate,” says Morrison.
But some melanoma cells gain a defense, if they cruise the body’s lymphatic system before settling down. In the lymph, their membranes pick up monounsaturated fatty acids that can’t react with iron in the same way, helping them resist ferroptosis, the researchers reported.
That’s not all. Melanoma cells that were the most efficient at metastasis rewired their metabolism, the scientists found. As a result, they gorged on a molecule called lactate in their surroundings, and they seemed to use this lactate to manufacture protective, oxidant-fighting molecules. When the scientists blocked the ability of the melanoma cells to suck up this lactate, metastatic disease in the mice was reduced.
In contrast, when they treated mice with more antioxidants, metastasizing cells were more likely to survive in the bloodstream and other organs — in some treated mice, the numbers of metastatic cells cruising the bloodstream more than doubled.
That result, published in 2015, was a huge surprise, says Morrison: “People think of antioxidants as being good for you.” Well, in his lab mice, antioxidants were good for cancer cells too — really good. The Washington Post called the study “terrifying,” “provocative” and “alarming.”
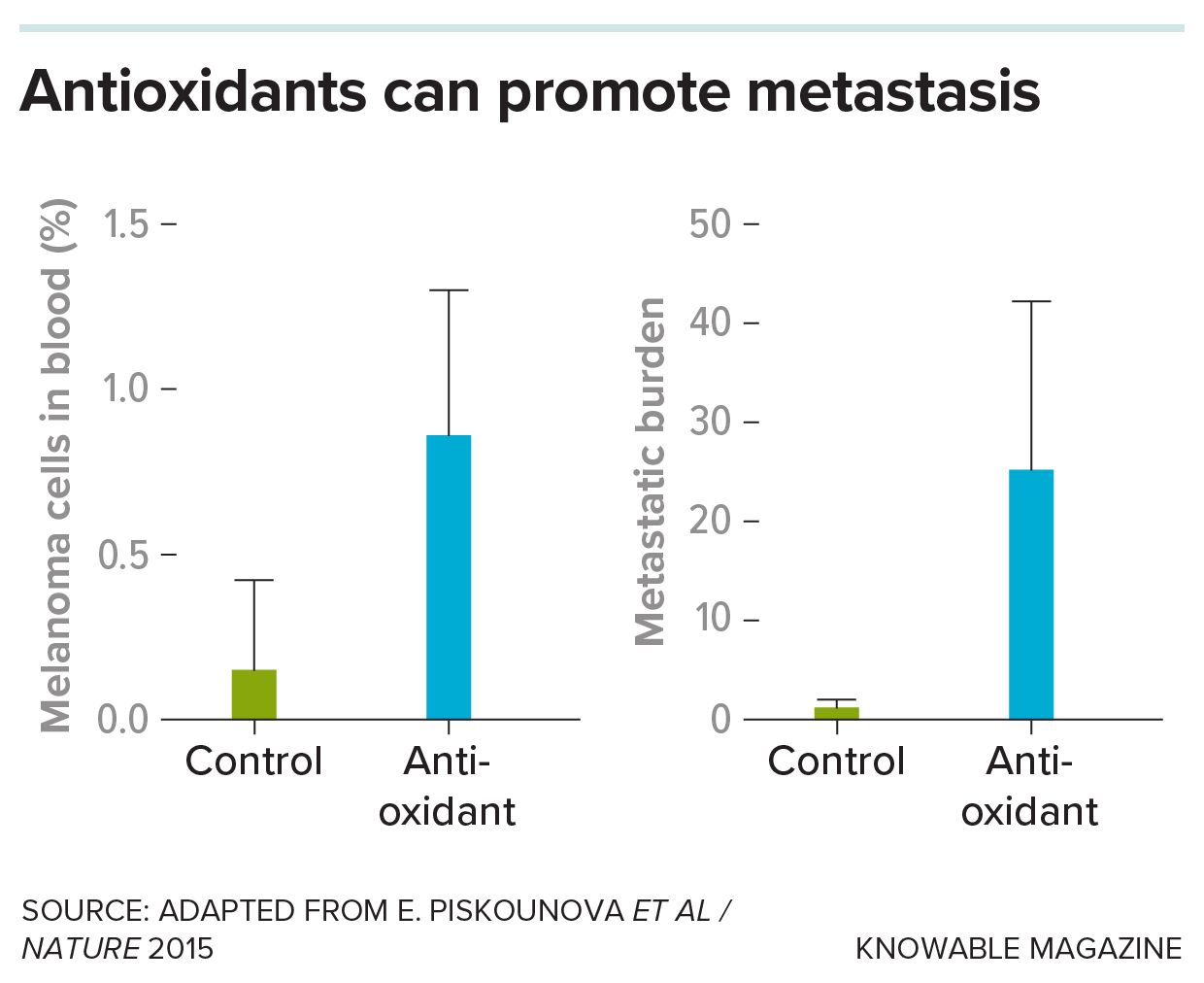
In an experiment, scientists studied a line of mice that had melanoma injected under their skin. Treatment with an antioxidant greatly increased the fraction of cells in blood that were metastasizing melanoma cells (left), as well as the burden — quantity — of metastatic cancer cells in their internal organs (right).
But the results do jive with past trials of antioxidants in cancer patients. In studies spanning decades, antioxidants such as beta-carotene and vitamin E were linked to increased lung cancer rates and deaths in smokers and higher prostate cancer rates in healthy men. Although those studies did not focus on metastatic cancer, Morrison sees a connection. “The reality is that at certain key phases of the evolution of cancer, the cancer cells are just on the edge of dying of oxidative stress, so they benefit more from the antioxidants than the normal cells do,” he speculates.
If antioxidants are good for cancers, then boosting reactive oxygen molecules might fight some kinds of metastasis. Indeed, some current cancer treatments do amplify reactive oxygen molecules to kill cancers.
These results imply that diet choices or supplements might influence cancer and metastasis risk. For example, Morrison speculates that a diet high in polyunsaturated fatty acids might lead to more of those pro-ferroptosis fatty acids in the membranes of cancer cells. If the cells are already quite vulnerable, a bit of polyunsaturated fat might be another way to nudge them over the cliff to cell death. For once, that’s an easy diet to swallow: One menu item might be salmon seared in soybean oil, Morrison suggests.
Dietary change is not going to vanquish cancer on its own, Fendt says. But, she adds, it might slow progression or help other treatments to work — although as the antioxidant trials illustrate, the effects of diet can be tricky to predict.
“It’s important to have really solid and rigorous science on those questions,” cautions Fendt. Some trials are underway — but, for now, there’s no “anti-metastasis” diet to prescribe.
10.1146/knowable-032725-1
TAKE A DEEPER DIVE | Explore Related Scholarly Articles