We are family: Tracing the evolution of animals
To understand the origins of multicelled life, researchers are studying a motley assortment of simpler animal relatives. The commonalities they’re unearthing offer a trove of clues about our mutual past.
Support sound science and smart stories
Help us make scientific knowledge accessible to all
Donate today
Once upon a time, there were a bunch of one-celled microbes, swimming, eating, reproducing, doing all the things that a one-cell bit of life can do.
Then, some time later, there were their descendants, the early animals: multicelled creatures, still swimming, eating, reproducing, but doing it all as teams of cells.
Exactly what happened between those points is a nigh-unfathomable mystery. But that in no way stops scientists from wondering and hypothesizing and investigating how the transition, about 600 million years ago, might have gone down.
The question is an old one, but researchers have made great progress in the past two decades, thanks to the genetic sequencing of single-celled life forms that are animals’ closest kin. It turns out that the unicellular ancestors of animals, way back then, were already remarkably well-equipped to take on teamwork. They probably could adopt a variety of cell shapes and do a number of jobs that came in handy for multicellularity. In fact, they might even have acted as groups, rather than single cells, from time to time.
“They were experimenting with multicellularity,” says Iñaki Ruiz-Trillo, an evolutionary biologist at the Institute of Evolutionary Biology in Barcelona, Spain.
And at some point, that experiment became permanent.
Advantages of upsizing
It was an experiment more than 3 billion years in the making. The earliest living cells appeared about 3.5 billion years ago, and some of them took a big step on the route to animals approximately 2 billion years ago when they added a nucleus in which to store their DNA. These nucleated microbes spawned complex, multicellular lineages several times during evolutionary history, creating fungi, plants and algae. They also gave rise to animals some 600 million or so years ago.
The vast majority of living things have held to a unicellular lifestyle for billions of years, with excellent evolutionary success, so cellular teamwork is hardly guaranteed to arise and far from certain to provide a superior lifestyle. “Multicellularity takes time and energy and resources to develop,” says Thibaut Brunet, an evolutionary cell biologist at the Institut Pasteur in Paris. But for those life forms that made the leap and made it stick, multicellularity presumably offered advantages that outweighed the costs.
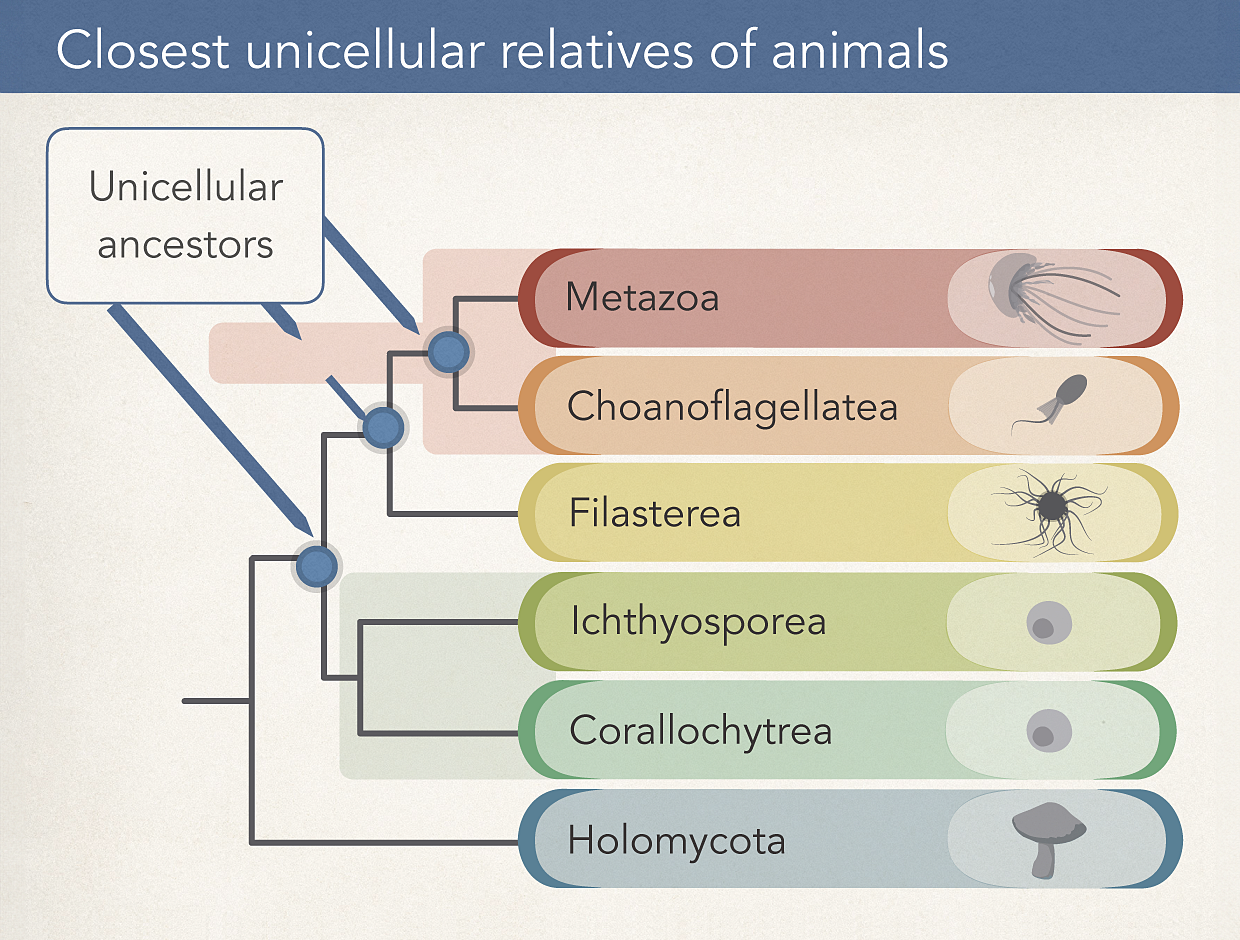
This evolutionary tree shows Metazoa, the group that includes most animals, along with four unicellular cousins and the Holomycota, which includes fungi, as an outgroup for comparison.
CREDIT: ADAPTED FROM IÑAKI RUIZ-TRILLO
Size, for one, was probably a factor. Being big means there are more things you can eat, and fewer things that can eat you. Multicellularity also allows you to have different parts that do different things at the same time: nerves to think, muscles to move, a stomach to digest food, and so on.
Researchers speculate that something major must have happened in the world to make teaming up such a good deal. “It must be environmental, to a large degree,” says Will Ratcliff, an evolutionary biologist at Georgia Tech in Atlanta. Two major kinds of Earthly changes stand out as possibilities.
One is the end of the Snowball Earth phase of the planet. This was an age, from roughly 710 to 640 million years ago, with at least two periods when the Earth was, if not wholly icy, at least pretty slushy. A warming climate might have somehow created an opportunity for multicellularity to evolve, Ruiz-Trillo suggests.
Another possibility is changes to Earth’s oxygen levels. These were initially much lower than today; early microbes did not require oxygen as modern animals do. The first time the planet’s oxygen supply swelled, during the Great Oxygenation Event about 2.4 billion years ago, was when cyanobacteria released oxygen as part of their photosynthesis. To the microbes of the time, the highly reactive gas was poison, and it decimated life forms. A second oxygenation event, of less certain causation, happened between about 850 and 540 million years ago, and this one may have set the stage for the emergence of animals.
Oxygen levels matter to large creatures that need oxygen, Ratcliff says, because the vital gas can diffuse only so far into tissues. For modern animals, the solution is circulatory systems that deliver oxygen through the body. The first big multicellular things, presumably, had not yet evolved such systems. The more oxygen there was on the outside, the more of it could diffuse deep into tissues, and the easier it would be to grow big.
Whatever pressures or opportunities induced our ancestor to commit to multicellularity, it did take that route — but who was “it”? That remains a mystery: Its cells were soft, squishy things that didn’t leave many fossils. “We don’t know what it looked like, and I don’t honestly think we ever will,” says Ratcliff. “It’s very difficult to try to reverse-engineer something that happened the better part of a billion years ago.”
Great-great-grandmama was a one-cell wonder
Still, researchers are trying. To hypothesize what the creatures might have been like, scientists like Ruiz-Trillo take a family-reunion kind of approach: Say you held a giant gathering, inviting all your cousins, second cousins, once-removeds, etc. Then, suppose you lined up all the relatives who share a common ancestor — say, the same great-great-great-grandmother — and looked for common features. If all those cousins have freckles and dimples, say, you could guess that great-great-great-grandmama, too, probably had them.
Scientists do similar studies, albeit of a more technical nature, comparing the genomes of animals with those of our very distant cousins. This is a strange family reunion indeed: In addition to the vast diversity of animals, including some of the earliest groups to branch off the animal family tree, the sponges and comb jellies, scientists know of four sets of one-celled cousins.
Most closely related to animals are the choanoflagellates. Found in fresh and salty waterways, they use whiplike tails called flagella to swim around or to waft bacteria toward them for supper. Rounding out the family reunion are the amoebalike or flagellated filastereans, the ichthyosporeans that often parasitize fish, and the amoeboid or immobile corallochytreans (also known as pluriformeans).
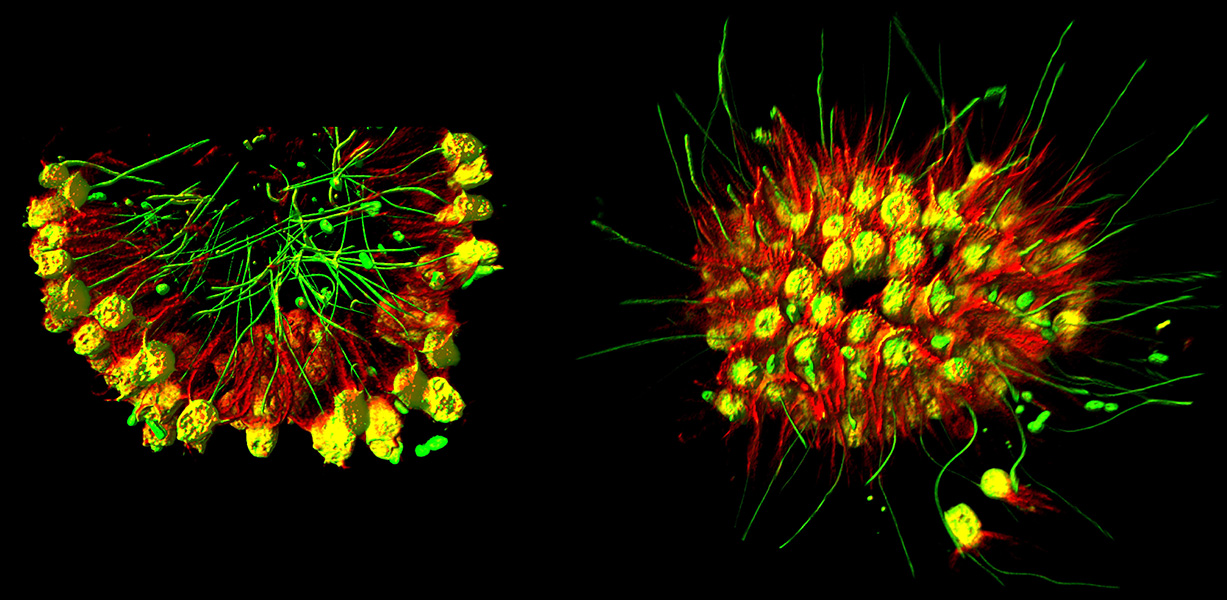
The choanoflagellate Choanoeca flexa can group together into colonies, with the tail structures either pointing in or out. The colonies switch to the tail-out shape in response to sudden darkness. These relatives of multicellular animals were discovered in splash pools on the Caribbean island of Curaçao and were christened “flexa” for the bendy sheet-like structures they formed.
CREDIT: THIBAUT BRUNET
None of these things, as they exist today, are the ancestor of animals. Rather, our lineage and theirs all split off from some common ancestor around 600 million years ago, and we’ve all evolved since then. But certain trait similarities between us and them suggest that our long-lost ancestor may have had those traits too.
Over the last decade, scientists have sequenced the genomes of 15 of those distant relatives. And therein lay surprises. These single-cell oddities contain almost as many genes as people, says Brunet, including DNA codes once thought to be animal exclusives. The critters have genes related to ones that make integrins and cadherins, proteins that help animal cells stick to each other. They have genes for control agents that guide cell identity, akin to the factors that tell an animal cell to be a brain cell or a muscle cell or a stomach cell. And they have genes involved in cell-to-cell communication.
In other words, these critters seem to be pre-adapted for multicellularity, and therefore our shared ancestor probably was too. But what would a unicellular pre-animal, all by its lonesome, have been doing with these kinds of genes?
In one-celled life forms, proteins like cadherins could have been useful because they are adhesive. “I compare them to Velcro,” says Jordi Paps, an evolutionary biologist at the University of Bristol, England. For a unicellular being, adhesive molecules could be a great way to capture passing bacteria for dinner.
Genes that govern cell identity may have helped the single-celled ancestors of animals take different forms at different times. All four close cousins of animals do this. Filastereans, for example, can adopt an amoeba-like shape, with long arms, that can divide into daughter cells, but they can also take on an armless, nondividing form.
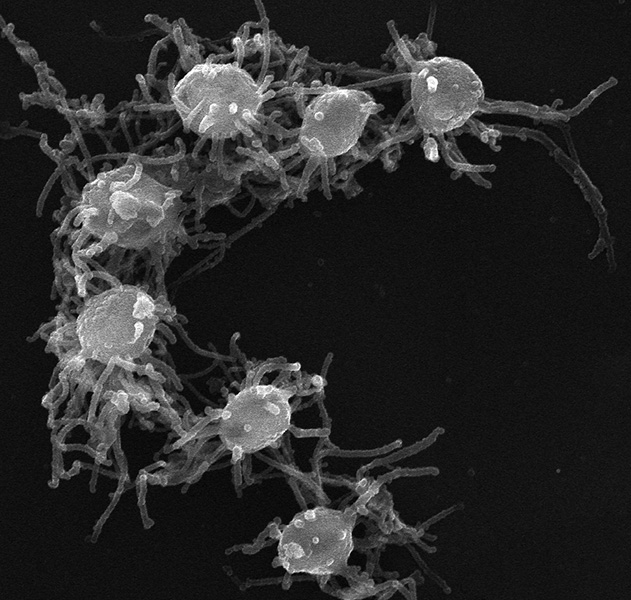
Capsaspora owczarzaki is a filasterean originally found inside a freshwater snail in Puerto Rico. It can take on multiple shapes, including an amoeba-like form with fingerlike projections it uses to attach to surfaces.
CREDIT: MULTICELLGENOME LAB
Finally, all four close cousins of animals have times in their lives when they flirt with multicellularity. Some choanoflagellates can make colonies by not fully separating from each other when they divide. At least one filasterean can aggregate once-separated cells. Corallochytreans live with two or so nuclei for a least a little while, while ichthyosporeans divide repeatedly to make a big ball of dozens or hundreds of nuclei before suddenly exploding into separate entities. That suggests the animal precursor, too, may have transiently grouped cells together. It’s not certain why this was beneficial; scientists speculate that the cells might have gained protection by acting in a herd, or traveled or hunted en masse.
Ruiz-Trillo and colleagues suggest the single-celled pre-animal was undergoing serious makeovers during its life cycle. Maybe sometimes it was like an amoeba, able to crawl around seeking food, and maybe other times it grew a flagellum to swim. Perhaps it sometimes survived alone, and other times grouped cells together.
But at any given time, our one-cell cousins can hold only one form, and scientists expect that was probably true of the pre-animal ancestor too. If it was dividing, it probably wouldn’t be able to swim, or to eat, says Brunet. It had to pick. What this means is that even in a single-celled creature, division of labor could have existed and conferred a benefit — but it was managed over time, not space.
Corallochytrium limacisporum exist as single cells with one or more nuclei. This free-living species was first discovered in lagoons of islands in the Arabian Sea.
CREDIT: MULTICELLGENOME LAB / FLICKR
Scientists think a trick to multicellularity was to repurpose the genes that were responsible for those temporal changes to work across space instead, controlling the shapes and jobs that cells do in different body parts. Such gene recycling, or “co-option,” has happened again and again during evolution. Beetles, for example, create their horns with genes co-opted from the genesis of other appendages, such as legs and antennae. Similarly, fish fins for swimming were co-opted so animals could walk on land, and leaves for photosynthesis were co-opted by cacti as spiny defenses. Same genes, different functions.
Acting like animals
To further investigate the multicelled mystery, a key step is to move beyond genetic comparisons and examine the biology of the one-celled kin of animals, says Omaya Dudin, an evolutionary cell biologist at the University of Geneva. Exploring ichthyosporeans, his group found striking similarities to animal development. When ichthyosporeans create their big balls of nuclei and then separate them, the cells divide in a way that looks a lot like cells dividing in an early animal embryo (like an insect embryo in one species, like a frog or mouse embryo in another).
That doesn’t necessarily mean the shared ancestor of bugs, mammals and ichthyosporeans also performed embryo-like cell division, Dudin says. It could be that the common ancestor just had enough of the necessary genes and abilities for their descendants to evolve remarkably similar division processes.
Still, it does mean that even something that seems very animal-specific, like embryonic division, isn’t necessarily animal-unique.
Ichthyosporeans generate structures with many nuclei before separating into individual cells. This species, Sphaeroforma arctica, was found in an Arctic crustacean.
CREDIT: MULTICELLGENOME LAB / FLICKR
In fact, even having multiple cell types at the same time might not be an exclusively animal feature. Observing the multi-nucleate balls of the ichthyosporean Chromosphaera perkinsii, Dudin’s team noted something surprising: There were two kinds of cells, one with flagella and one without. Researchers have also spotted evidence of multiple cell types — most round, but some oblong — in choanoflagellate colonies. That suggests, just maybe, that the precursor of animals might have had moments not just of multicellularity but also of division of cellular labor.
Altogether, it seems clear that our unicellular ancestors were ready for multicellularity. While the act of simply teaming up could have happened quickly, “there was probably a lot of complexity to the origin of animals,” says Brunet. “The full extent of animal multicellular complexity certainly arose over thousands, and most probably millions, of years.”
There are still plenty of details for scientists to iron out: Did the pre-animal look like choanoflagellates, as one popular theory attests, or perhaps adopt a variety of forms at different times? Were the first animals like sponges, as traditionally presumed, or maybe more like comb jellies?
Ruiz-Trillo, who coauthored a look at the roots of multicellularity in the 2023 Annual Review of Microbiology, refuses to name a favorite hypothesis: “I think we don’t have enough data to say,” he says.
To get more data, he’ll have to invite more creatures to the family reunion. And he is busy doing just that, seeking as yet unknown microbes from aquatic sites around the world. So far, he’s identified at least eight new groups of animal cousins by analyzing their genes alone. If scientists can find, grow and study these creatures, they’ll likely find new clues to the origin of multicellular animal-kind.
It should be quite a party.
10.1146/knowable-093024-1
TAKE A DEEPER DIVE | Explore Related Scholarly Articles